Knowledge
Automated Pharmacopoeia Testing of Purified Water & Water For Injection

Pharmaceutical laboratory’s goal is to operate their TOC analysis with the lowest amount of invalid results
Automated Pharmacopoeia Testing of PurifiedWater & Water For Injection
The requirements for Total Organic Carbon (TOC) analysis of Water for Injection (WFI) and pharmaceutical purified water (PW) are provided for in monographs of the United States Pharmacopoeia (USP) and European Pharmacopoeia (EP). The principle behind these methods is to challenge the instrument oxidation efficiency by comparing the recovery of a standard solution (rs) and the system suitability solution (rss). The response of the water used to create these solutions, reagent water (rw), is subtracted from each of these solutions’ responses to yield a corrected response. A maximum carbon concentration for the reagent water is limited to100 parts per billion of carbon (ppb C). From the corrected response results, the response efficiency (E) of the analysis is calculated. According to the pharmacopoeias, the value of E is required to be within 85% - 115% for the instrument to be suitable for TOC analysis on PW and WFI samples (see Equations 1-3). This paper outlines the features of a modern TOC analyzer that imroves the longevity of a laboratory’s system suitability performance through unattended automated performance.
R1 = rs–rw
R1 = corrected standard solution response
rs = standard solution response rw = reagent water response
Equation 1. Corrected Standard Solution Response is the Limit Response
R2 = rss – rw
R2 = corrected system suitability solution
response rss = system suitability solution response rw = reagent water response
Equation 2. Corrected System Suitability Solution Response
E = (R2 / R1) * 100
E = % response efficiency
R2 = corrected system suitability solution response
R1 = corrected standard solution response
Equation 3. Response Efficiency
Automated Instrument Features to Calibration
Teledyne Tekmar’s Fusion TOC analyzer user friendly software, TekLinkTM, is more powerful and easier to navigate than ever before. TekLinkTM has multiple user defined method parameters that allow the end user to customize the instrument for their specific sample needs. The Fusion provides superior analytical analysis for a variety of sample applications. For the USP / EP system suitability test, the Fusion utilizes a default pharmaceutical TOC method that provides the best performance for water-for-injection (WFI), ultra-low purified water. Additionally, this method’s robust characteristics are strong enough to handle the most challenging cleaning validation samples.
The Fusion analyzer has an integrated autosampler with four center stock solution positions that can hold 125 mL bottles, Figure 1. Unattended multiple runs of the system suitability test reagent water, standard solution, and challenge solution can be analyzed by placing 125 mL bottles in the center positions A, B, C or D of the autosampler. This feature allows the system suitability test to be run at multiple intervals from the center positions; thus, allowing extra available sample positions in the autosampler for sample vials. By utilizing larger sample container to increase system suitability frequency, less risk is incurred of invalid sample results. Further laboratory efficiency and reduction of risk can be achieved by using USP and NIST certified pre-made TOC standards, reagents and purified water.

The software of older TOC analyzers required additional software, such as spreadsheets to complete system suitability calculations. Hence, the process required multiple sets and data transfer to perform compendial analysis. Unlike older software, TekLinkTM Software has automated pass / fail alerts for the system suitability performance. If a result is out of specification set by the end-user, then the software can automatically recalibrate, halt or continue the scheduled analysis. A typical system suitability analysis autosampler schedule and report for the Fusion TOC Analyzer is shown in Table 1
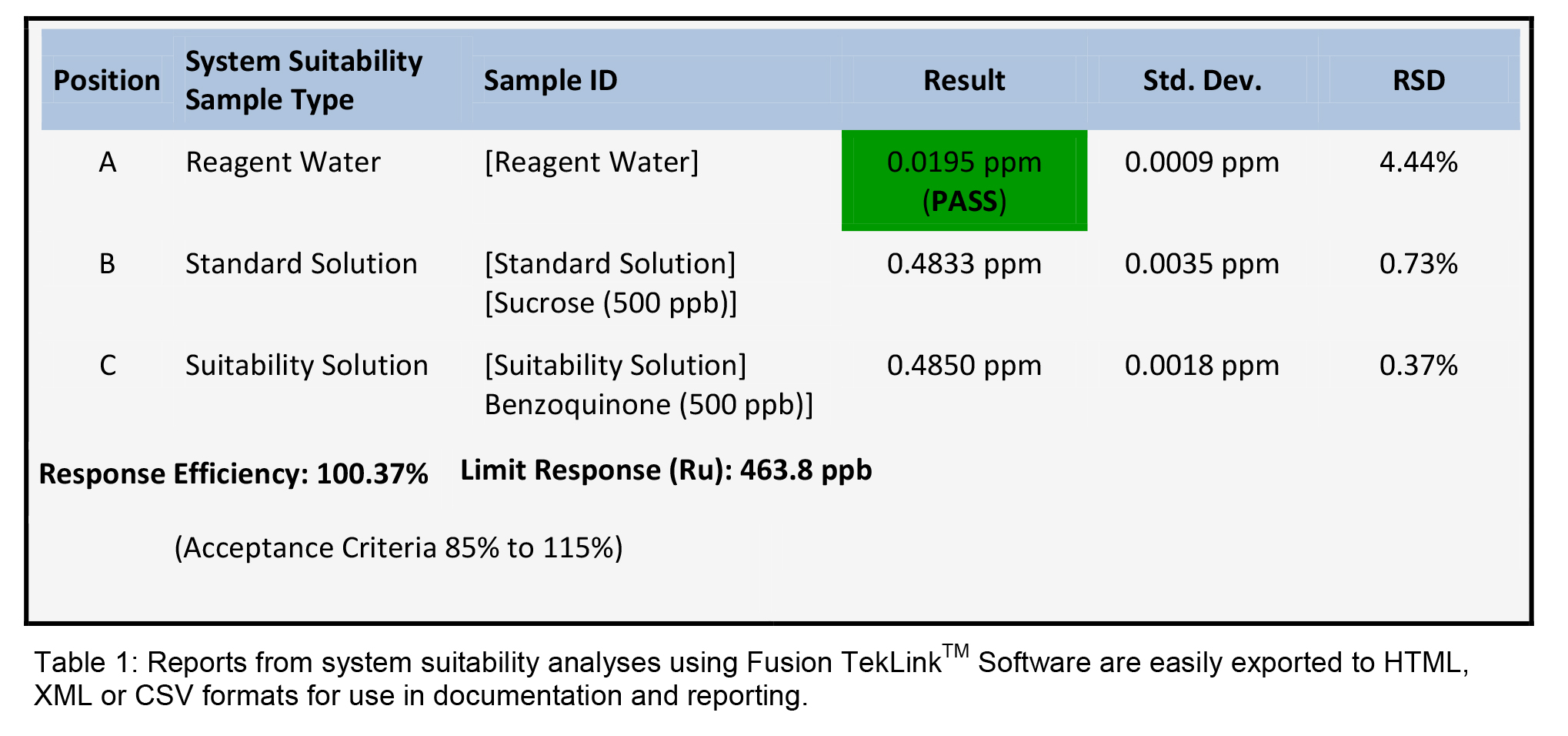
For ease of calibration and calibration verification, the Fusion can utilize a fourth 125mL center stock solution position. From one stock solution, 13 calibration points can be attained through auto-dilution using TekLinkTM Software. By utilizing the center positions for calibrations and verifications, additional sample position are available for WFI, purified water and cleaning validation analysis.
Improving System Suitability Testing and TOC Analysis
Every pharmaceutical laboratory’s goal is to operate their TOC analysis with the lowest amount of invalid results. By utilizing the latest technology in automation and reporting, the Fusion TOC analyzer provides unique features that save time and increase laboratory throughput making the task of system suitability performance analysis easier for compliance monitoring. Additionally, successful system suitability result details along with all meta-data (calibration curve, method, electronic signatures and audit trail details) are documented within the sample analysis report.
Credit : Teledyne Tekmar
Contact us
388/5 Nuanchan Road, Nuanchan,
Buengkum, Bangkok 10230
0 2363 8585 (auto)
0 2363 8595
081 498 9939






