Knowledge
Total Organic Carbon Analysis for Purified Water and Water for Injection

Purified Water (PW) and Water for Intravenous Injection (WFI) the implications on human health require very strict regulation.
Total Organic Carbon Analysis for Purified Water and Water for Injection
Abstract
In pharmaceuticals, water, specifically Purified Water (PW) and Water for Intravenous Injection (WFI), is vital for drug preparation. Producing and ensuring the cleanliness of this water entails very strict quality control and precise analytical testing methods. The United States Pharmacopeia (USP) and Japanese Pharmacopeia (JP) have promoted Total Organic Carbon (TOC) analysis as the procedure to verify PW and WFI are free of TOCs and therefore able to be used for pharmaceutical purposes. A TOC system is considered suitable, under the USP, if it can recover 90% of a 0.5mgC/L system suitability standard, 1,4-benzoquinone. The JP requirement uses a dodecylbenzenesulfonic acid standard with a 90% recovery criterion.
This application note will present data following these strict criteria. In addition, this study will verify the suitability of Teledyne Tekmar’s TOC Analyzer (Figure 1) for analysis of TOC at 0.05 mgC/mL, as opposed to the current standard of 0.5 mgC/L.

Introduction
In pharmaceuticals, water, specifically Purified Water (PW) and Water for Intravenous Injection (WFI) is vital for the preparation of intravenous drugs. Since these medications are normally introduced directly into the bloodstream, the reagents used to make them need to be as clean as possible. The United Stated Pharmacopeia (USP) and Japanese Pharmacopeia (JP) lay out strict regulations that mandate that PW and WFI are free of Total Organic Carbon (TOC) to be used for pharmaceutical applications. These methods also demand extremely sensitive instruments to detect TOC at the levels they require.
The JP test method for TOC specifies, “The instrument should be capable of measuring the amount of organic carbon down to 0.050 mgC/L.”1 Also, the system should be capable of generating not less than 0.450 mgC/L when using a 0.806 mg/L solution of dodecylbenzenesulfonic acid as a sample. This solution has a carbon concentration of 0.500 mgC/L and the JP method specifies that the instrument must be capable of oxidizing 90% (0.450 mgC/L) of this solution as a measured sample.
This application note will show the capability of the Teledyne Tekmar Fusion TOC analyzer of meeting these two requirements set forth in Japanese Pharmacopeia test method for total organic carbon.
Experimental-Instrument Conditions
For this study, analysis was performed on the Teledyne Tekmar Fusion, a UV/Persulfate TOC Analyzer using the default Japanese Pharmacopeia method (Table 1).
Sample Preparation
KHP (Potassium Hydrogen Phthalate) and dodecylbenzenesulfonic acid were used as sources of TOC. A stock solution of KHP was prepared at 1000 mgC/L in a 1 L volumetric flask. From this KHP stock solution, serial dilutions were made to prepare 0.500 mgC/L and 5.00 mgC/L working solutions. A 50 mgC/L stock solution of dodecylbenzenesulfonic acid was prepared by dissolving 80.6 mg/L of dodecylbenzenesulfonic acid into deionized water. A 0.500 mgC/L working solution was prepared by diluting this stock.
Results
A. Recovery of Dodecylbenzenesulfonic Acid
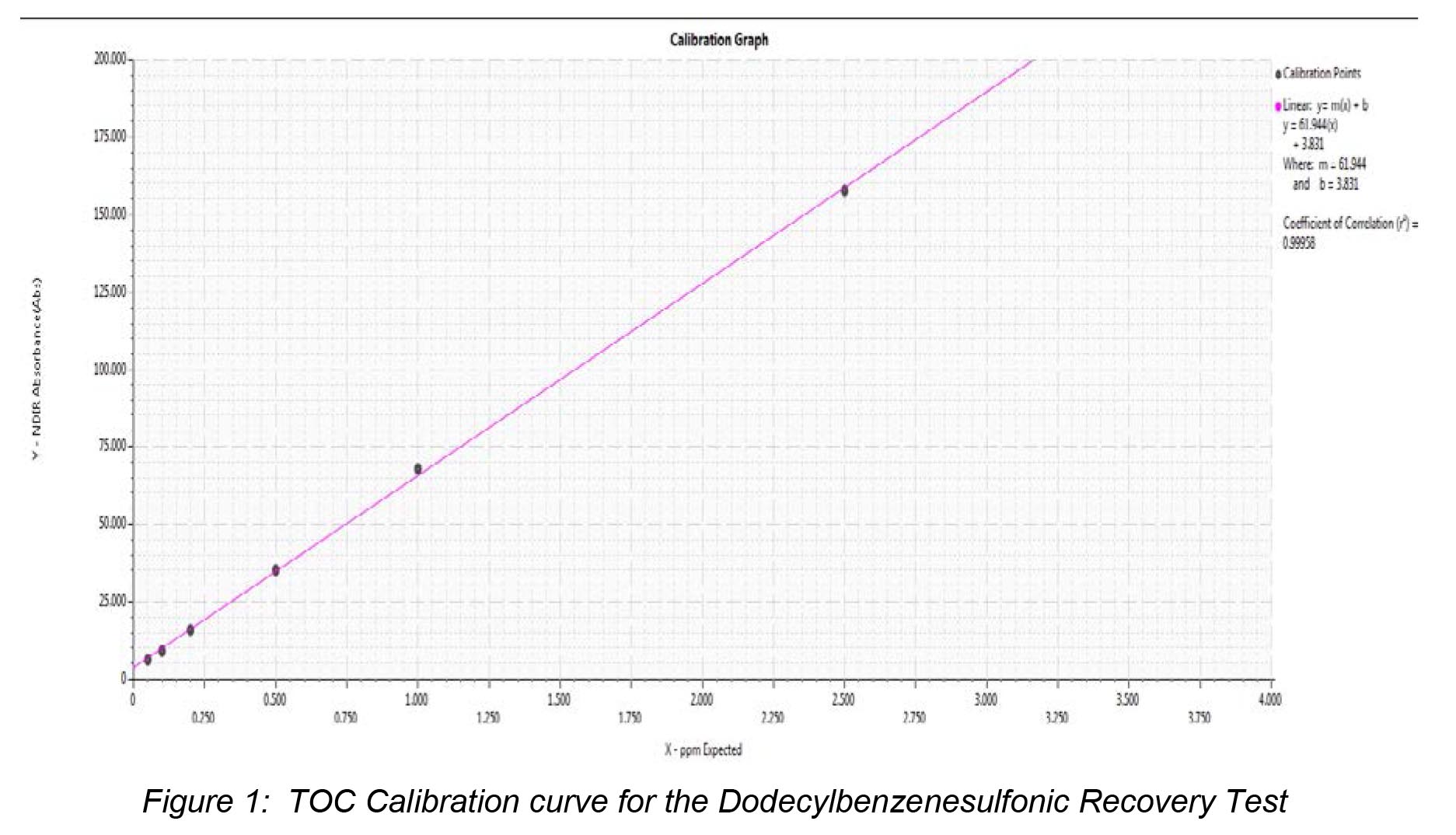
The Japanese Pharmacopeia mandates that the system “should be capable of generating not less than 0.450 mg/L of carbon when using the dodecylbenzenesulfonic acid as the sample.”1 The Fusion TOC analyzer was calibrated using a 5.00 mgC/L KHP stock solution utilizing the auto-dilution feature. The regression factor (r2) for the curve was determined to be 0.9995 over a calibration range of 0.050-2.50 mgC/L. Figure 2 illustrates the calibration curve developed for this study. A sample of dodecylbenzenesulfonic acid with a carbon concentration of 0.500 mgC/L was analyzed as a sample. The results from this analysis can be seen in Table 2.
Credit : Teledyne Tekmar
Contact us
388/5 Nuanchan Road, Nuanchan,
Buengkum, Bangkok 10230
0 2363 8585 (auto)
0 2363 8595
081 498 9939






