Hits: 5465
EPR
Hits: 5465

Hits: 5465
Electron Paramagnetic Resonance (EPR)
Bruker Optics เสนอความหลากหลายของเครื่อง FTIR Spectroscopy (ฟูเรียทรานสฟอร์มอินฟาเรด สเปกโตรสโคปี) สำหรับงานวิเคราะห์ทั่วไป
• ALPHA เป็น FT-IR spectrometer ที่ถูกออกแบบให้มีขนาดเล็ก ทนต่อแรงสั่นสะเทือน และสามารถใช้งานง่าย ส่วนประกอบที่มีคุณภาพและการออกแบบที่ทันสมัย ทำให้มั่นใจในผลที่ถูกต้องและแม่นยำ ที่ได้จากการวัดด้วยเวลาอันสั้น ALPHA ถูกนำไปใช้งานอย่างกว้างขวาง ในงานตรวจสอบคุณภาพของผลิตภัณฑ์ในอุตสาหกรรมต่างๆ
• TENSOR เป็น FT-IR spectrometer ที่เหมาะสำหรับงานที่มีความซับซ้อน รวมถึงมีประสิทธิภาพที่โดดเด่น ในส่วนของ sensitivity และค่าใช้จ่ายของผู้ประกอบการ เนื่องจากเครื่องมือทำมาจากวัสดุที่มีคุณภาพสูง จึงทำให้อายุการใช้งานยาวนาน TENSOR II นำไปใช้งานได้หลากหลาย และสามารถขยายงานได้โดยการเชื่อมต่อ FT-IR กับเทคนิคอื่นๆ เช่น FT-IR microscope HYPERION, micro plate reader HTS-XT, thermal gravimetric analysis (TGA) หรือ gas chromatography (GC) นอกจากนี้ TENSOR II ยังสามารถวิเคราะห์หาโปรตีนและสารละลายด้วยการต่อ TENSOR II กับ CONFOCHECK
• LUMOS เป็นเครื่อง FT-IR microscope แบบอัตโนมัติ โดยได้รวมเอาความสามารถในด้านของการตรวจสอบของภาพและการวิเคราะห์ด้วยแสงอินฟราเรด สำหรับตัวอย่างที่มีระดับไมครอน LUMOS จึงเป็นเครื่องมือที่ผู้ใช้งานจะใช้งานได้อย่างสะดวกสบาย
• Mobile-IR เป็นเครื่อง FT-IR แบบพกพา สำหรับงานที่ต้องออกนอกสถานที่
• แผ่นไมโคร HTS-XT สำหรับ FT-IR
Bruker Optics offers a wide variety of laboratory FT-IR Spectroscopy for routine applications.
• The ALPHA is an extremely compact FT-IR spectrometer with rugged and intuitive design. Quality components and state-of-the-art technology ensure accurate and precise results within short measurement time. The ALPHA is widely used in quality control of industrially manufactured products.
• TENSOR II is the right tool for more advanced applications in your laboratory. It is characterized by an outstanding performance for highest sensitivity and a low cost of ownership due to high quality components with long lifetime. TENSOR II offers flexibility and expandability for unlimited in-compartment sampling and coupling technology; e.g. with the FT-IR microscope HYPERION, the micro plate reader HTS-XT, thermal gravimetric analysis (TGA) or gas chromatography (GC). A dedicated configuration of theTENSOR II is available for the investigation of proteins and aqueous solutions (CONFOCHECK).
• LUMOS is a fully automated stand-alone FT-IR microscope. It combines best performance for visual inspection and infrared spectral analysis of micro samples with highest comfort in use.
• The Mobile-IR is a portable FT-IR spectrometer for routine applications outside of the laboratory.
• The microtiter plate extension HTS-XTenables high throughput screening for infrared spectroscopy.
• ALPHA เป็น FT-IR spectrometer ที่ถูกออกแบบให้มีขนาดเล็ก ทนต่อแรงสั่นสะเทือน และสามารถใช้งานง่าย ส่วนประกอบที่มีคุณภาพและการออกแบบที่ทันสมัย ทำให้มั่นใจในผลที่ถูกต้องและแม่นยำ ที่ได้จากการวัดด้วยเวลาอันสั้น ALPHA ถูกนำไปใช้งานอย่างกว้างขวาง ในงานตรวจสอบคุณภาพของผลิตภัณฑ์ในอุตสาหกรรมต่างๆ
• TENSOR เป็น FT-IR spectrometer ที่เหมาะสำหรับงานที่มีความซับซ้อน รวมถึงมีประสิทธิภาพที่โดดเด่น ในส่วนของ sensitivity และค่าใช้จ่ายของผู้ประกอบการ เนื่องจากเครื่องมือทำมาจากวัสดุที่มีคุณภาพสูง จึงทำให้อายุการใช้งานยาวนาน TENSOR II นำไปใช้งานได้หลากหลาย และสามารถขยายงานได้โดยการเชื่อมต่อ FT-IR กับเทคนิคอื่นๆ เช่น FT-IR microscope HYPERION, micro plate reader HTS-XT, thermal gravimetric analysis (TGA) หรือ gas chromatography (GC) นอกจากนี้ TENSOR II ยังสามารถวิเคราะห์หาโปรตีนและสารละลายด้วยการต่อ TENSOR II กับ CONFOCHECK
• LUMOS เป็นเครื่อง FT-IR microscope แบบอัตโนมัติ โดยได้รวมเอาความสามารถในด้านของการตรวจสอบของภาพและการวิเคราะห์ด้วยแสงอินฟราเรด สำหรับตัวอย่างที่มีระดับไมครอน LUMOS จึงเป็นเครื่องมือที่ผู้ใช้งานจะใช้งานได้อย่างสะดวกสบาย
• Mobile-IR เป็นเครื่อง FT-IR แบบพกพา สำหรับงานที่ต้องออกนอกสถานที่
• แผ่นไมโคร HTS-XT สำหรับ FT-IR
Bruker Optics offers a wide variety of laboratory FT-IR Spectroscopy for routine applications.
• The ALPHA is an extremely compact FT-IR spectrometer with rugged and intuitive design. Quality components and state-of-the-art technology ensure accurate and precise results within short measurement time. The ALPHA is widely used in quality control of industrially manufactured products.
• TENSOR II is the right tool for more advanced applications in your laboratory. It is characterized by an outstanding performance for highest sensitivity and a low cost of ownership due to high quality components with long lifetime. TENSOR II offers flexibility and expandability for unlimited in-compartment sampling and coupling technology; e.g. with the FT-IR microscope HYPERION, the micro plate reader HTS-XT, thermal gravimetric analysis (TGA) or gas chromatography (GC). A dedicated configuration of theTENSOR II is available for the investigation of proteins and aqueous solutions (CONFOCHECK).
• LUMOS is a fully automated stand-alone FT-IR microscope. It combines best performance for visual inspection and infrared spectral analysis of micro samples with highest comfort in use.
• The Mobile-IR is a portable FT-IR spectrometer for routine applications outside of the laboratory.
• The microtiter plate extension HTS-XTenables high throughput screening for infrared spectroscopy.

EMXnano
EMXnano - The New Standard for Bench-Top EPR
Giving you the power and flexibility you really need in an easy to use compact enclosure, the EMXnano brings affordable highend EPR performance to labs for the first time, delivering a real choice of analysis options.
For the first time in this instrument category quantitative EPR is offered at your fingertip, thanks to a fully calibrated instrument and inclusion of the Bruker patented spin counting module.
Routine Ease of Use with Unmatched Performance
Extending Bruker’s renowned EMX spectrometer family to the bench-top class, the EMXnano is a completely new development featuring the latest digital and microwave technologies. Combined with a new generation of magnet system with full range up to 6.5 kG and a highly efficient microwave resonator, this state-of-the-art bench-top instrument is superior in sensitivity and stability, making it ideal for a comprehensive range of analysis and teaching applications.
The new dedicated bench-top instrument requires minimal infrastructure with a low cost of ownership, making it suitable for a wide range of laboratory types.
Sensitivity and Stability
No matter what the focus of your application is, the crucial required strengths of an EPR spectrometer are sensitivity and stability. The latest generation magnet and microwave technology delivers class leading performance, enabling the EMXnano to combine both ease of use with highest quality EPR data.
Any customer can confidently add, or enhance, their lab with real world EPR:
EPR in chemistry
Giving you the power and flexibility you really need in an easy to use compact enclosure, the EMXnano brings affordable highend EPR performance to labs for the first time, delivering a real choice of analysis options.
For the first time in this instrument category quantitative EPR is offered at your fingertip, thanks to a fully calibrated instrument and inclusion of the Bruker patented spin counting module.
Routine Ease of Use with Unmatched Performance
Extending Bruker’s renowned EMX spectrometer family to the bench-top class, the EMXnano is a completely new development featuring the latest digital and microwave technologies. Combined with a new generation of magnet system with full range up to 6.5 kG and a highly efficient microwave resonator, this state-of-the-art bench-top instrument is superior in sensitivity and stability, making it ideal for a comprehensive range of analysis and teaching applications.
The new dedicated bench-top instrument requires minimal infrastructure with a low cost of ownership, making it suitable for a wide range of laboratory types.
Sensitivity and Stability
No matter what the focus of your application is, the crucial required strengths of an EPR spectrometer are sensitivity and stability. The latest generation magnet and microwave technology delivers class leading performance, enabling the EMXnano to combine both ease of use with highest quality EPR data.
Any customer can confidently add, or enhance, their lab with real world EPR:
- Integrated, motorized reference standard (marker) for amplitude and g-factor
- Reference free concentration determination of paramagnetic species (SpinCount)
- Identification of paramagnetic species by spectrum simulation and fitting (SpinFit)
- Spectral library including spin traps
- Dedicated work flows for measurement and analysis
EPR in chemistry
Enzyme Reactions
Detection and study of the active site of Cu,Zn-SOD.
Many enzyme reactions involve one-electron oxidation steps with formation of paramagnetic transient state of the enzyme detectable by EPR. The paramagnetic center where the unpaired electron is located, is usually centered at a transition metal (metalloproteins) or is an amino acid derived radical. Detection and identification of the paramagnetic centers is important to understand the function of the enzymes. For example, in the native SOD1 enzyme, the active site contains one Cu(II) ion that gives a very characteristic EPR spectrum.
Many enzyme reactions involve one-electron oxidation steps with formation of paramagnetic transient state of the enzyme detectable by EPR. The paramagnetic center where the unpaired electron is located, is usually centered at a transition metal (metalloproteins) or is an amino acid derived radical. Detection and identification of the paramagnetic centers is important to understand the function of the enzymes. For example, in the native SOD1 enzyme, the active site contains one Cu(II) ion that gives a very characteristic EPR spectrum.
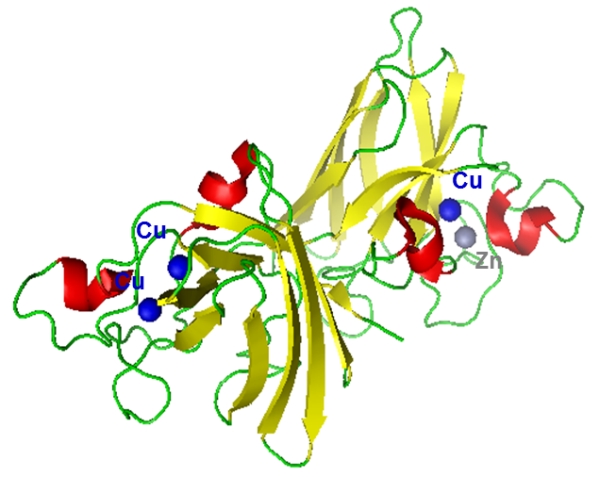
Crystal structure (1E9P.pdb) of Cu(II)-SOD protein
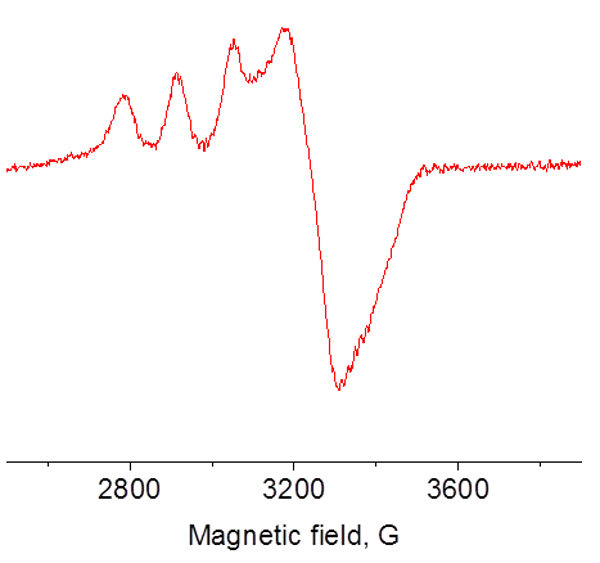
EPR spectrum of Cu(II)-SOD protein at 77 K using finger dewar
Reaction Kinetics
Kinetics analysis of vitamin C antioxidant ability.
Many chemical reactions involve the transfer of one electron. Each electron transfer results in an unpaired electron creating paramagnetic free radicals. EPR is the ideal spectroscopic technique to measure these species as well as to monitor the time behavior of their creation and disappearance. EPR solely has the ability to detect free radicals unambiguously. For example, antioxidants such as vitamin C are important in neutralizing dangerous free radicals in living things and kinetics indicates their effectiveness.
Many chemical reactions involve the transfer of one electron. Each electron transfer results in an unpaired electron creating paramagnetic free radicals. EPR is the ideal spectroscopic technique to measure these species as well as to monitor the time behavior of their creation and disappearance. EPR solely has the ability to detect free radicals unambiguously. For example, antioxidants such as vitamin C are important in neutralizing dangerous free radicals in living things and kinetics indicates their effectiveness.
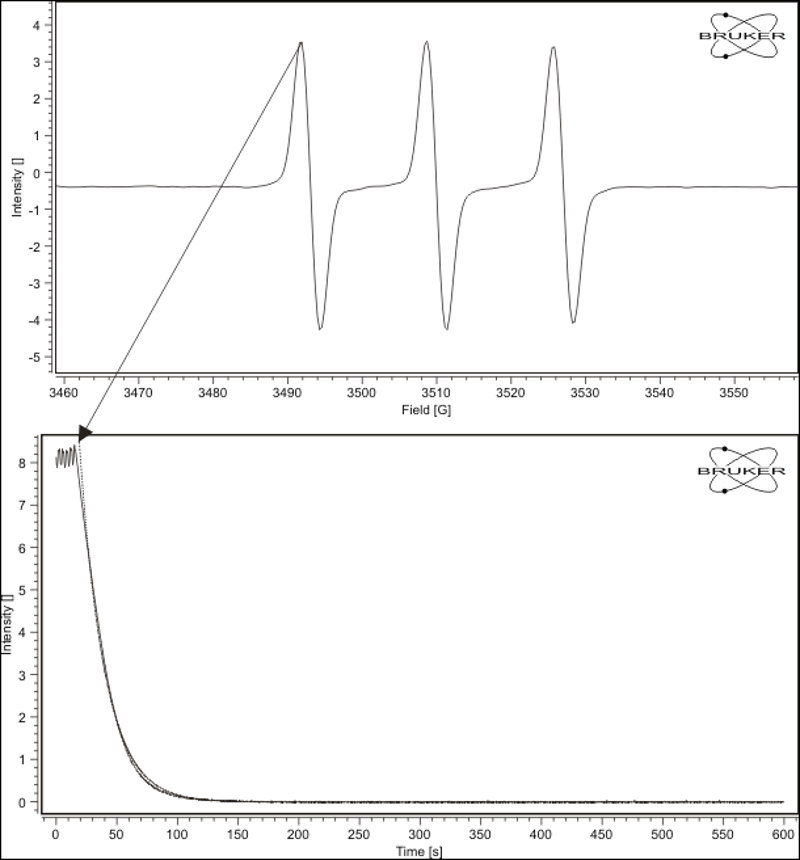
Experimental data of the reduction of the nitroxide TEMPOL by ascorbate (vitamin C)
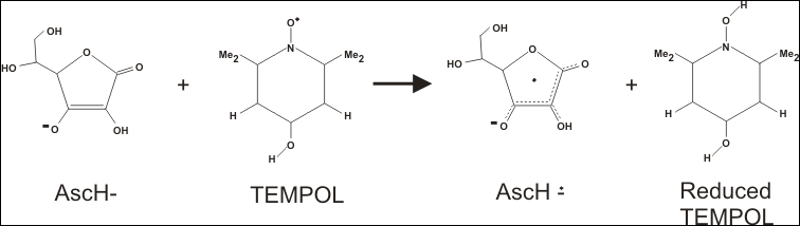
Mechanism of TEMPOL reduction by vitamin C
Photochemistry
Light degradation of hops in beer.
The majority of photochemical reactions take place through free radical formation as intermediates. For example, hops used in the brewing process contain a mixture of active components that include humulones, cohumulones, adhumulones, beta acids and essential oils. Some forms of these components are photo-active. Light exposure of beer leads to the formation of free radicals that combines with sulfur compounds and gives unpleasant flavor and odor to the beer.
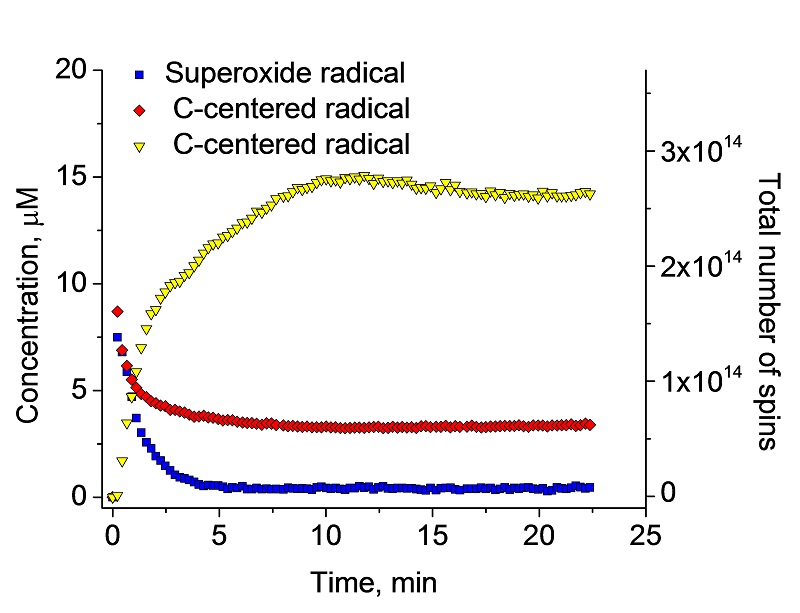
The hop product was exposed to UV/Vis light from 220-600 nm using UV lamp accessory. The light-induced free radicals were recorded in the presence of the spin trap DMPO and identified as superoxide anion radical and two C-centered radicals.
The majority of photochemical reactions take place through free radical formation as intermediates. For example, hops used in the brewing process contain a mixture of active components that include humulones, cohumulones, adhumulones, beta acids and essential oils. Some forms of these components are photo-active. Light exposure of beer leads to the formation of free radicals that combines with sulfur compounds and gives unpleasant flavor and odor to the beer.
The hop product was exposed to UV/Vis light from 220-600 nm using UV lamp accessory. The light-induced free radicals were recorded in the presence of the spin trap DMPO and identified as superoxide anion radical and two C-centered radicals.

Catalysis
Hydroxyl radical generation through photocatalytic reaction of TiO2.
The modern chemical industry relies heavily on homogeneous and heterogeneous catalysts. Understanding the operational mode, or reactivity, of these catalysts is crucial for improved developments and enhanced performance. Where paramagnetic centers are involved, ranging from transition metal ions to defects and radicals, EPR spectroscopy is without doubt the technique of choice. For example, photocatalytic oxidation of organic pollutants is frequently carried out using semiconducting polycrystalline powders such as TiO2. A hydroxyl radical is easily formed by light irradiation of TiO2 and detected by EPR using spin traps.
The modern chemical industry relies heavily on homogeneous and heterogeneous catalysts. Understanding the operational mode, or reactivity, of these catalysts is crucial for improved developments and enhanced performance. Where paramagnetic centers are involved, ranging from transition metal ions to defects and radicals, EPR spectroscopy is without doubt the technique of choice. For example, photocatalytic oxidation of organic pollutants is frequently carried out using semiconducting polycrystalline powders such as TiO2. A hydroxyl radical is easily formed by light irradiation of TiO2 and detected by EPR using spin traps.
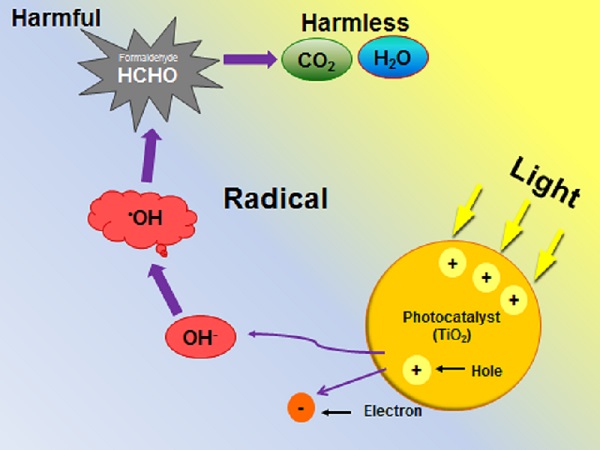
Mechanism of hydroxyl radical formation upon light irradiation of TiO2
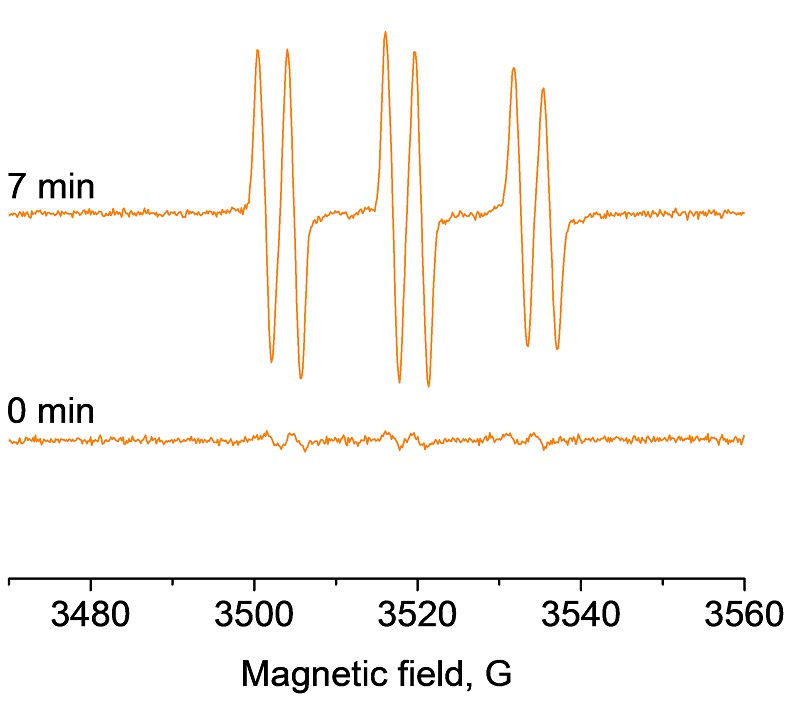
EPR spectra obtained upon irradiation of aqueous TiO2 suspensions in the presence of spin trapping agent PBN
Electrochemistry
EPR electrochemistry study of ruthenium complexes.
Electrochemical generation method combined with EPR has been used to identify and investigate free radicals derived from both organic and inorganic compounds. Inorganic dyes can be used to improve the efficiency of solar cells. In order to optimize the ligands, one must understand the electronic structure of the dye. Here the electrochemistry and EPR combined with DFT calculations and UV/Vis spectroscopy show the unpaired electron is delocalized between the metal and the ligand.
Electrochemical generation method combined with EPR has been used to identify and investigate free radicals derived from both organic and inorganic compounds. Inorganic dyes can be used to improve the efficiency of solar cells. In order to optimize the ligands, one must understand the electronic structure of the dye. Here the electrochemistry and EPR combined with DFT calculations and UV/Vis spectroscopy show the unpaired electron is delocalized between the metal and the ligand.
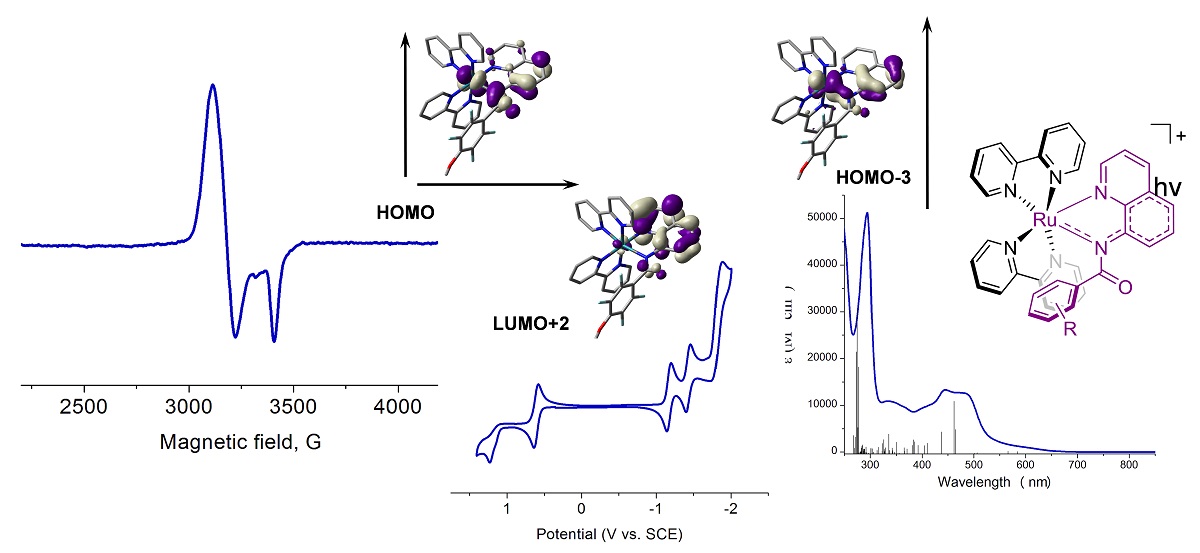
Data courtesy of Prof. J. Rochford, University of Massachusetts Boston (Inorg. Chem., 2016, 55 (5), pp 2460–2472)
Redox Chemistry
Enzymatic activity of SOD protein studied via Cu(II) reduction.
Enzymes in the human body regulate oxidation-reduction reactions. These complex proteins, of which several hundred are known, act as catalysts, speeding up chemical processes in the body. Oxidation-reduction reactions also take place in the metabolism of food for energy, with substances in the food broken down into components the body can use. For example, the dismutase activity of Cu,Zn-SOD protein involves reduction of Cu(II)-SOD to Cu(I)-SOD:
Enzymes in the human body regulate oxidation-reduction reactions. These complex proteins, of which several hundred are known, act as catalysts, speeding up chemical processes in the body. Oxidation-reduction reactions also take place in the metabolism of food for energy, with substances in the food broken down into components the body can use. For example, the dismutase activity of Cu,Zn-SOD protein involves reduction of Cu(II)-SOD to Cu(I)-SOD:
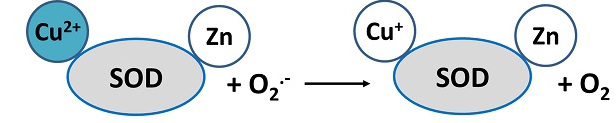
Reduction of Cu(II)-SOD (EPR active) to Cu(I)-SOD (EPR inactive)
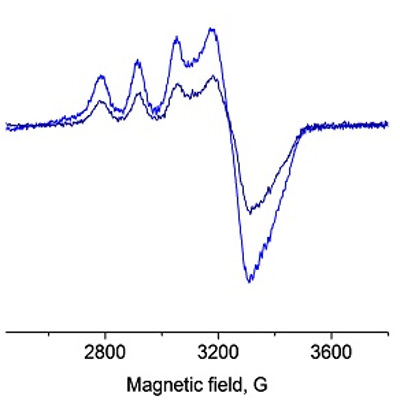
Cu(II)-SOD has a very characteristic EPR signal which decays upon reduction of Cu(II) -> Cu(I).
Antioxidants
Detection of ascorbate radical upon oxidation of vitamin C.
The delicate balance between the advantageous and detrimental effects of free radicals is one of the important aspects of human (patho)physiology. Imbalanced generation of toxic radicals is highly correlated with the pathogenesis of many diseases which require the application of selected antioxidants to regain the homeostasis. EPR is used to determine the oxidative status of biological systems using endogenous long-lived free radicals (ascorbyl radical, tocopheroxyl radical, melanin) as markers.
The delicate balance between the advantageous and detrimental effects of free radicals is one of the important aspects of human (patho)physiology. Imbalanced generation of toxic radicals is highly correlated with the pathogenesis of many diseases which require the application of selected antioxidants to regain the homeostasis. EPR is used to determine the oxidative status of biological systems using endogenous long-lived free radicals (ascorbyl radical, tocopheroxyl radical, melanin) as markers.
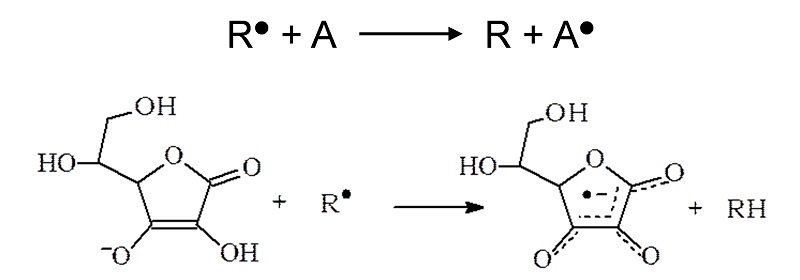
Reaction of a toxic radical R● with an antioxidant A. Also pictured is the reaction of the antioxidant ascorbic acid (Vitamin C) with a radical
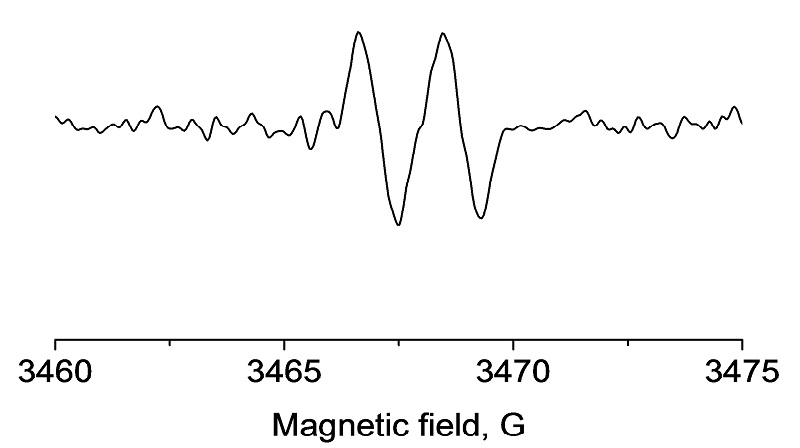
EPR spectrum of ascorbate (Vitamin C) radical
EPR in biology
RNA and DNA oxidation
DNA-derived radicals detected upon CuCl2/H2O2 treatment.
EPR spectroscopy in conjunction with spin trapping has been employed successfully to detect and identify high-molecular-weight species generated as a result of reactive oxygen species (ROS)-induced damage to biological macromolecules, such as DNAs and RNAs. The destruction or alteration of these materials is known to play a key role in a large number of cellular injuries and diseases.
EPR spectroscopy in conjunction with spin trapping has been employed successfully to detect and identify high-molecular-weight species generated as a result of reactive oxygen species (ROS)-induced damage to biological macromolecules, such as DNAs and RNAs. The destruction or alteration of these materials is known to play a key role in a large number of cellular injuries and diseases.
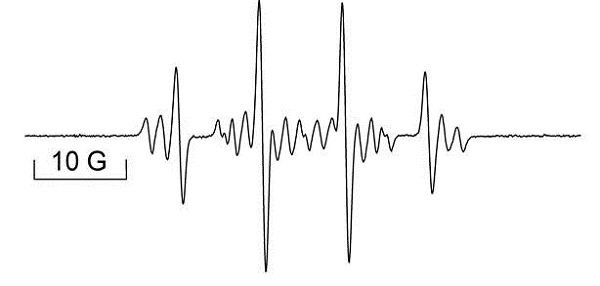
EPR spectrum of N-centered radical upon DNA damage after CuCl2/H2O2 treatment using DMPO as spin trap. Spectrum also consists of two other radicals that are not DNA-derived. Data courtesy of Dr. R. Mason, NIEHS (Free Radic. Biol. Med. 2011 50(11) pp 1536
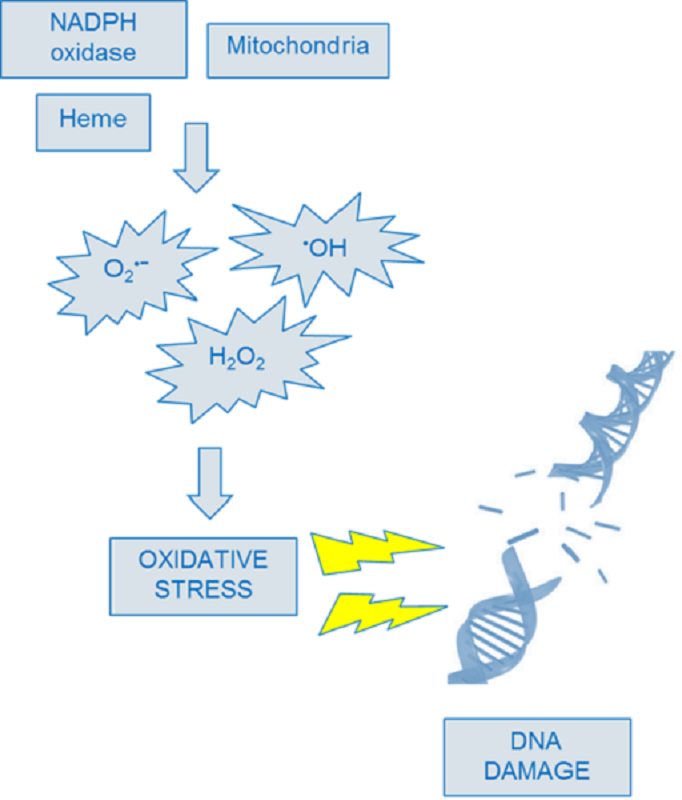
Mechanism of DNA damage by reactive oxygen species (ROS)
Screening DNP agents
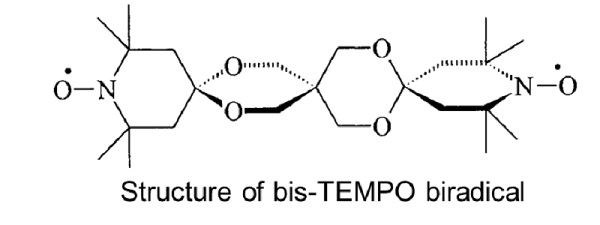
EPR spectrum and dipolar coupling determination of bis-TEMPO.
Correct concentration of DNP polarizing agents is crucial to the success of a DNP experiment. Samples can be pre-screened before DNP experiments using the patented SpinCount module, even in the MAS rotor. Relaxation times are critical for DNP efficiency therefore P1/2measurements at low temperature to estimate the DNP efficiencies of new polarization agents are invaluable. Another characteristic of importance in DNP measurements is the electron-electron dipolar coupling that is easily measured from solution and frozen solution EPR spectra.
Correct concentration of DNP polarizing agents is crucial to the success of a DNP experiment. Samples can be pre-screened before DNP experiments using the patented SpinCount module, even in the MAS rotor. Relaxation times are critical for DNP efficiency therefore P1/2measurements at low temperature to estimate the DNP efficiencies of new polarization agents are invaluable. Another characteristic of importance in DNP measurements is the electron-electron dipolar coupling that is easily measured from solution and frozen solution EPR spectra.
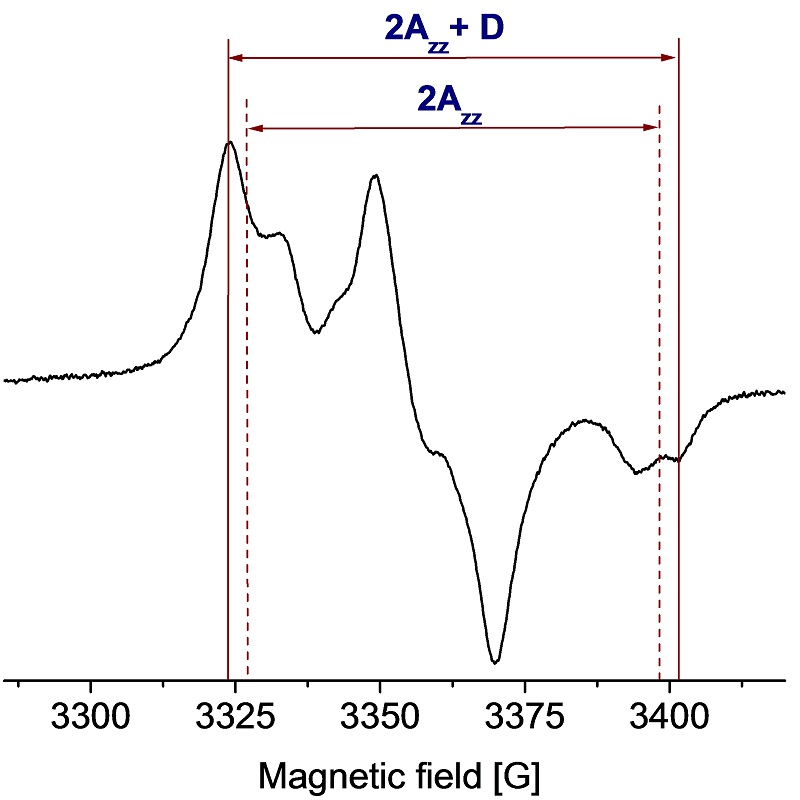
EPR spectrum of DNP agent (bis-TEMPO biradical)
D [G] = 18562/R3 [Å]
Dipolar coupling measured: D = 7.9 G.
Distance determined: R = 13 Å
Data courtesy of Prof. T. Prisner, University of Frankfurt
(Angew. Chem. Int. Ed., 2009, 48, 4996)
Dipolar coupling measured: D = 7.9 G.
Distance determined: R = 13 Å
Data courtesy of Prof. T. Prisner, University of Frankfurt
(Angew. Chem. Int. Ed., 2009, 48, 4996)
Enzyme Reactions
Detection and study of the active site of Cu,Zn-SOD.
Many enzyme reactions involve one-electron oxidation steps with formation of paramagnetic transient state of the enzyme detectable by EPR. The paramagnetic center where the unpaired electron is located, is usually centered at a transition metal (metalloproteins) or is an amino acid derived radical. Detection and identification of the paramagnetic centers is important to understand the function of the enzymes. For example, in the native SOD1 enzyme, the active site contains one Cu(II) ion that gives a very characteristic EPR spectrum.
Many enzyme reactions involve one-electron oxidation steps with formation of paramagnetic transient state of the enzyme detectable by EPR. The paramagnetic center where the unpaired electron is located, is usually centered at a transition metal (metalloproteins) or is an amino acid derived radical. Detection and identification of the paramagnetic centers is important to understand the function of the enzymes. For example, in the native SOD1 enzyme, the active site contains one Cu(II) ion that gives a very characteristic EPR spectrum.

Crystal structure (1E9P.pdb) of Cu(II)-SOD protein

EPR spectrum of Cu(II)-SOD protein at 77 K using finger dewar
EPR in biomedical
Nitric oxide
Binding of nitric oxide to oxyhemoglobin detected at 100 K.
Nitric Oxide (NO) is a highly reactive regulatory molecule which has many important physiological roles, such as a neurotransmitter in the central nervous system, a regulator of vasomotor tone in the cardiovascular system, and a cytotoxic mediator of the immune system. NO is a free radical and its short half-life (< 30 sec), has rendered direct measurement difficult. The instability of NO can be overcome by using a NO-trapping technique, in which a more stable complex is formed and subsequently detected by EPR. For example, the oxidation of nitric oxide (NO) to nitrate by oxyhemoglobin (oxyHb) is a fundamental reaction in NO biology and binding of NO to the heme can be characterized by EPR.
Nitric Oxide (NO) is a highly reactive regulatory molecule which has many important physiological roles, such as a neurotransmitter in the central nervous system, a regulator of vasomotor tone in the cardiovascular system, and a cytotoxic mediator of the immune system. NO is a free radical and its short half-life (< 30 sec), has rendered direct measurement difficult. The instability of NO can be overcome by using a NO-trapping technique, in which a more stable complex is formed and subsequently detected by EPR. For example, the oxidation of nitric oxide (NO) to nitrate by oxyhemoglobin (oxyHb) is a fundamental reaction in NO biology and binding of NO to the heme can be characterized by EPR.
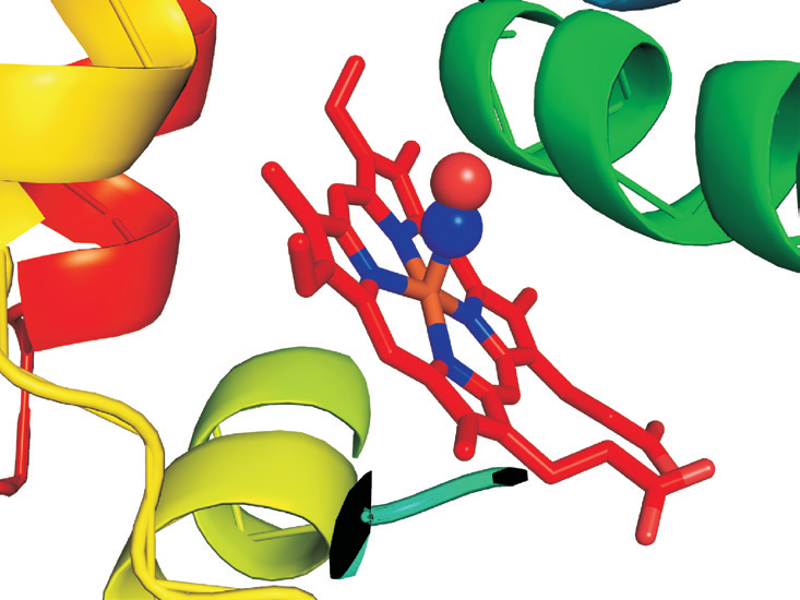
Crystal structure of NO-Hb (4G51.pdb)
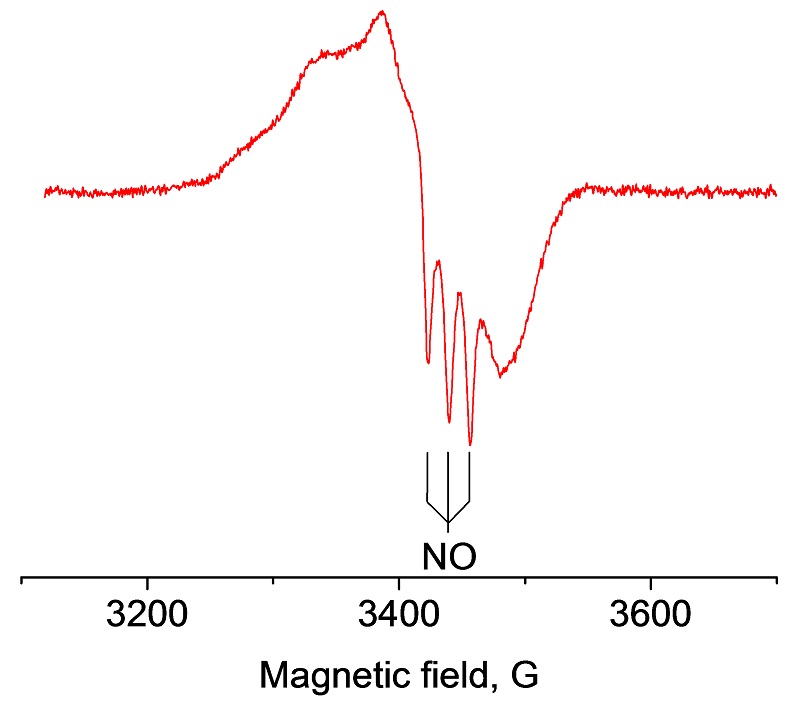
EPR spectrum of NO-Hb complex at 100 K with VT unit
Detection of Reactive Oxygen Species (ROS) using spin traps
Quantitative EPR analysis of superoxide and hydroxyl radicals.
Oxidative stress and damage in cells is associated with the development of cancer, Alzheimer‘s disease, atherosclerosis, autism, infections and Parkinson‘s disease. Reactive Oxygen Species (ROSs) are the main cause of oxidative stress and damage in cells, causing damage to proteins, lipids and DNA. Two leading ROS are radicals such as the superoxide radical (O2•-) and the hydroxyl radical (HO•) as shown here in the Xanthine/Xanthine oxidase system where their generation and decomposition can be accurately followed with the EMXnano.
Oxidative stress and damage in cells is associated with the development of cancer, Alzheimer‘s disease, atherosclerosis, autism, infections and Parkinson‘s disease. Reactive Oxygen Species (ROSs) are the main cause of oxidative stress and damage in cells, causing damage to proteins, lipids and DNA. Two leading ROS are radicals such as the superoxide radical (O2•-) and the hydroxyl radical (HO•) as shown here in the Xanthine/Xanthine oxidase system where their generation and decomposition can be accurately followed with the EMXnano.
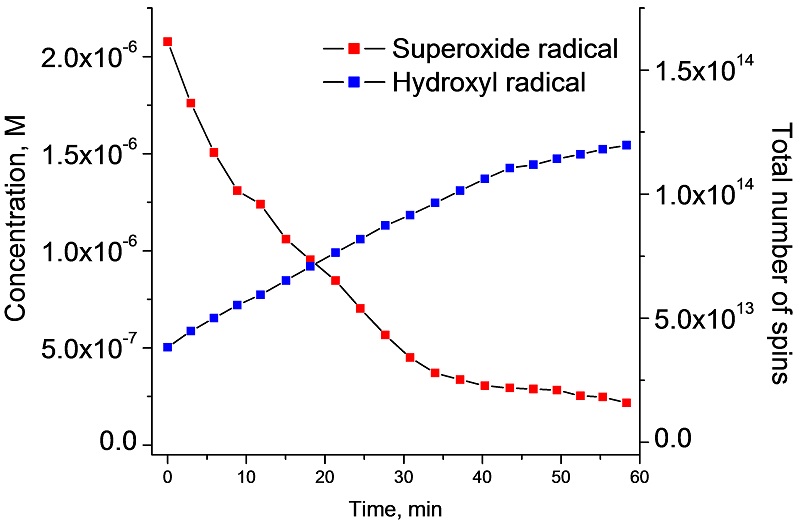
SpinCount provides a report showing the time evolution of the concentration of the radicals
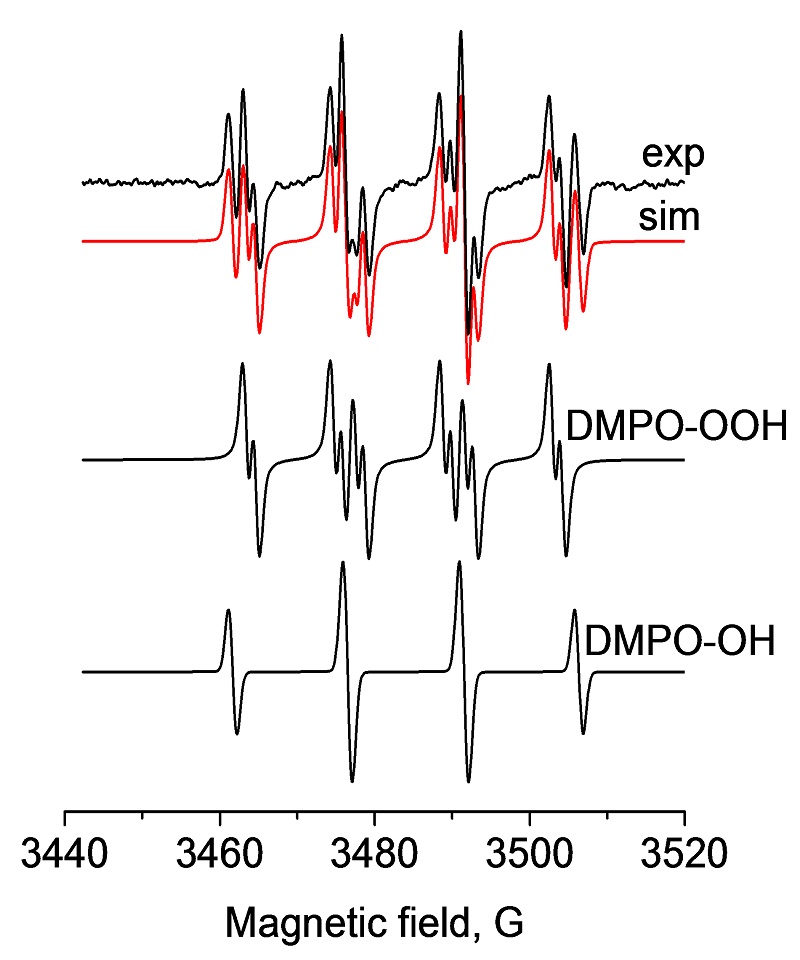
EPR spectra and SpinFit simulations of DMPO radical (superoxide and hydroxyl) adducts in xanthine/xanthine oxidase
Detection of Reactive Oxygen Species (ROS) using spin probes
Time course of superoxide formation using the spin probe CMH.
In vascular cells, increased generation of superoxide (O2•-) has been suggested to occur in hypertension, diabetes, and heart failure. Thus the accurate detection and ability to quantify O2•- are critically important in understanding the pathogenesis of these various cardiovascular disorders and other noncardiovascular diseases. As shown here the generation of superoxide over time can be easily monitored with the EMXnano.
In vascular cells, increased generation of superoxide (O2•-) has been suggested to occur in hypertension, diabetes, and heart failure. Thus the accurate detection and ability to quantify O2•- are critically important in understanding the pathogenesis of these various cardiovascular disorders and other noncardiovascular diseases. As shown here the generation of superoxide over time can be easily monitored with the EMXnano.
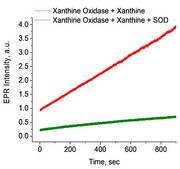
Detection of superoxide radical (O2•-) is confirmed by suppression of the EPR signal by superoxide dismutase (SOD)
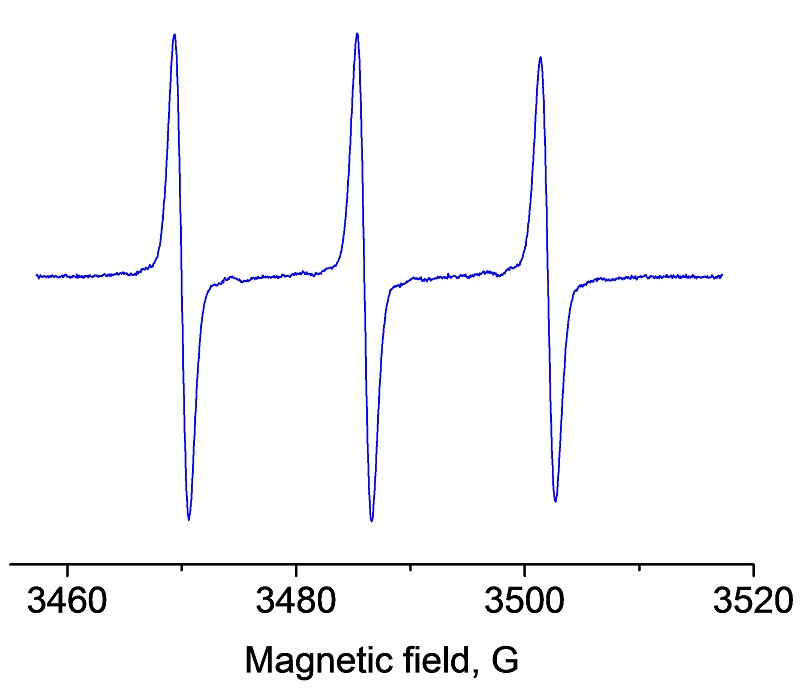
EPR spectrum of CM• nitroxide due to the reaction: CMH + O2•-–» CM• + H2O2
EPR in material science
Polymer degradation
HALS successfully prevents polymer photoxidation.
The degradation of polymers due to light exposure leads to discoloration of the polymer and a decrease in the mechanical properties (elasticity, toughness, etc). To prevent this decomposition, hindered amine light stabilizers (HALS) are added to the polymer. By monitoring the EPR signals of these light stabilizers, their effectiveness can be evaluated using the EMXnano.
The degradation of polymers due to light exposure leads to discoloration of the polymer and a decrease in the mechanical properties (elasticity, toughness, etc). To prevent this decomposition, hindered amine light stabilizers (HALS) are added to the polymer. By monitoring the EPR signals of these light stabilizers, their effectiveness can be evaluated using the EMXnano.
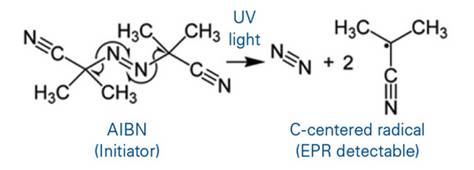
Photo initiation and radical formation upon light irradiation
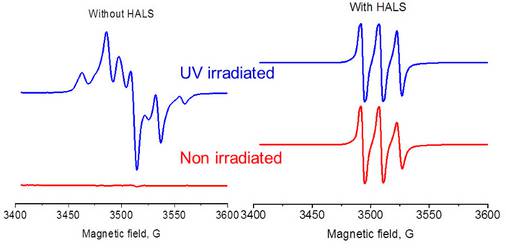
The EPR signal generated in the polymer during UV irradiation (left) is completely suppressed after addition of the HALS where only the HALS EPR spectrum is observed (right)
Polymer structure
EPR studies of polyelectrolyte multilayer (PEM) films using nitroxide spin labels.
Multilayers of polyelectrolytes (polymers bearing dissociated ionic groups) are formed by the alternating adsorption of oppositely charged polyelectrolytes, so called layer-by-layer technique. PEM films composed of strong polycation and weak polyanion that is usually spin labeled with free nitroxide (4-amino-TEMPO) are studied by EPR. The growth of the PEM films is monitored and quantitative EPR analysis provides information about each double layer.
Multilayers of polyelectrolytes (polymers bearing dissociated ionic groups) are formed by the alternating adsorption of oppositely charged polyelectrolytes, so called layer-by-layer technique. PEM films composed of strong polycation and weak polyanion that is usually spin labeled with free nitroxide (4-amino-TEMPO) are studied by EPR. The growth of the PEM films is monitored and quantitative EPR analysis provides information about each double layer.
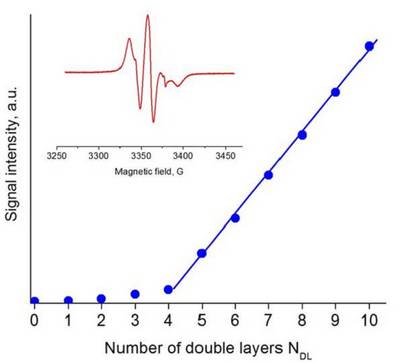
Signal intensity of polycation/TEMPO-labeled polyanion multilayer films in contact with buffer solution of pH 4 in dependence on number of double layers NDL.
Paint properties
HALS EPR signal in paint indicating deterioration after UV exposure.
The main cause of paint film deterioration is the degradation of several components, including the binder and certain pigments. This is caused by the formation of free radicals from prolonged exposure to UV light (sunlight), moisture and freeze-thaw cycles. Free radicals are highly reactive and either form or breakdown chemical bonds in substances. In the case of paint durability on exposure, free radicals actually damage the film. This process is very similar to how skin ages. Skin contains free radicals that, when exposed to years of sunlight, will show signs of aging, including wrinkling, peeling, sun spots and overall dryness.
The main cause of paint film deterioration is the degradation of several components, including the binder and certain pigments. This is caused by the formation of free radicals from prolonged exposure to UV light (sunlight), moisture and freeze-thaw cycles. Free radicals are highly reactive and either form or breakdown chemical bonds in substances. In the case of paint durability on exposure, free radicals actually damage the film. This process is very similar to how skin ages. Skin contains free radicals that, when exposed to years of sunlight, will show signs of aging, including wrinkling, peeling, sun spots and overall dryness.

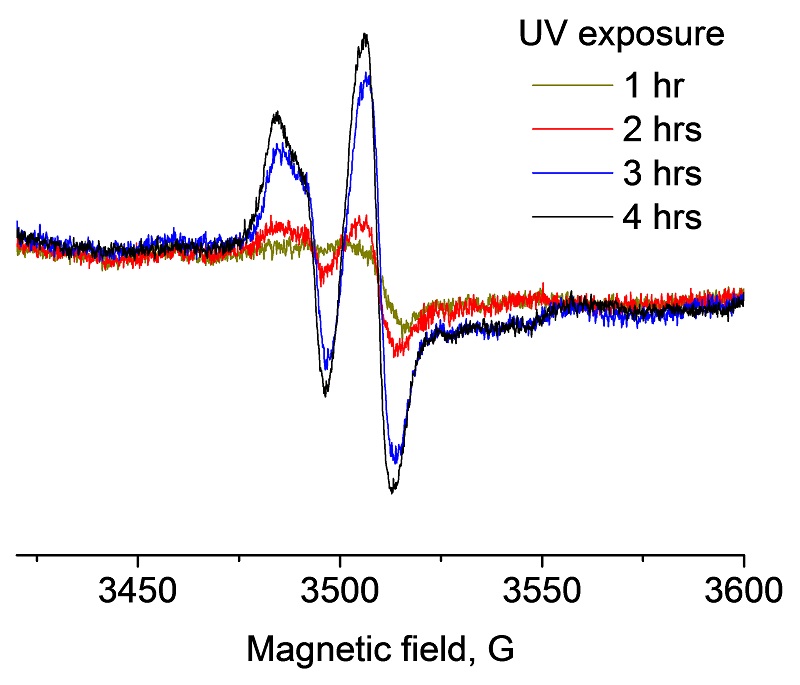
EPR spectra of HALS (hindered amine light stabilizers) detected in paint after UV exposure
Solar cells
Defects in amorphous silicon detected by EPR.
Silicon is the most common material for the production of solar cells in the photovoltaic industry either in mono- or polycrystalline form. Specific characterization of paramagnetic defects can be done by EPR to gain insight into how paramagnetic centers induced by degradation influence the efficiency of solar cell active layers. EPR studies on amorphous silicon photovoltaics demonstrated that a strong relationship exists between the presence of paramagnetic defects and the resulting charge collection efficiency in such material.
Silicon is the most common material for the production of solar cells in the photovoltaic industry either in mono- or polycrystalline form. Specific characterization of paramagnetic defects can be done by EPR to gain insight into how paramagnetic centers induced by degradation influence the efficiency of solar cell active layers. EPR studies on amorphous silicon photovoltaics demonstrated that a strong relationship exists between the presence of paramagnetic defects and the resulting charge collection efficiency in such material.
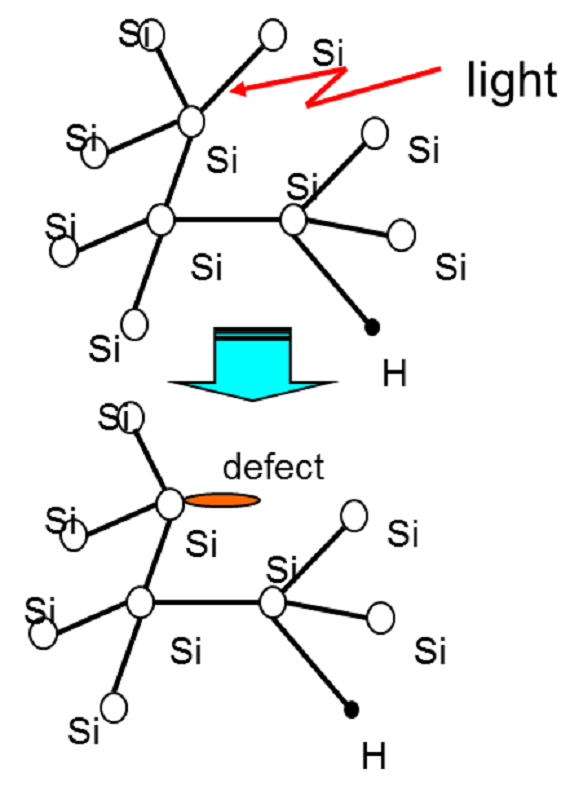
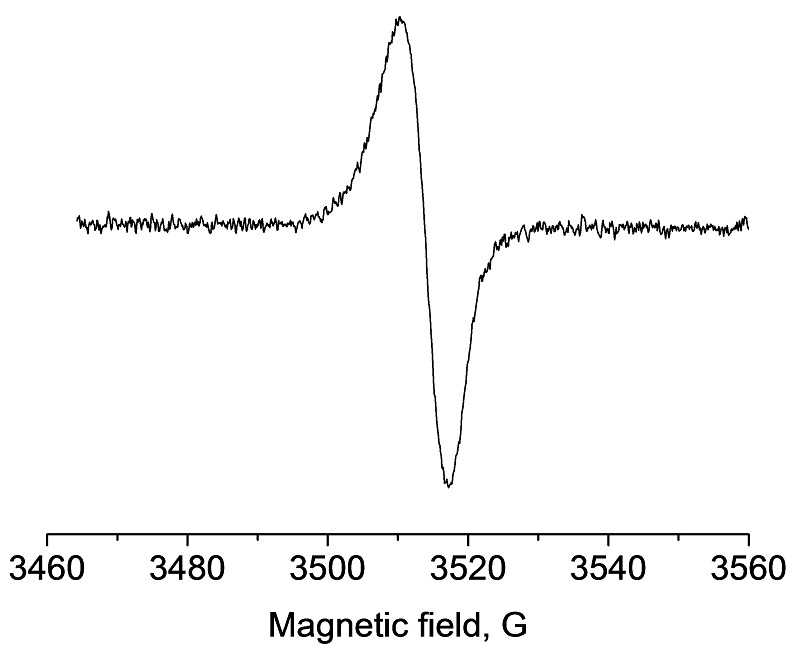
Light-induced defect in amorphous silicon detected by EPR due to breaking of weak Si-Si bonds
Screening DNP agents
EPR spectrum and dipolar coupling determination of bis-TEMPO.
Correct concentration of DNP polarizing agents is crucial to the success of a DNP experiment. Samples can be pre-screened before DNP experiments using the patented SpinCount module, even in the MAS rotor. Relaxation times are critical for DNP efficiency therefore P1/2 measurements at low temperature to estimate the DNP efficiencies of new polarization agents are invaluable. Another characteristic of importance in DNP measurements is the electron-electron dipolar coupling that is easily measured from solution and frozen solution EPR spectra.
Correct concentration of DNP polarizing agents is crucial to the success of a DNP experiment. Samples can be pre-screened before DNP experiments using the patented SpinCount module, even in the MAS rotor. Relaxation times are critical for DNP efficiency therefore P1/2 measurements at low temperature to estimate the DNP efficiencies of new polarization agents are invaluable. Another characteristic of importance in DNP measurements is the electron-electron dipolar coupling that is easily measured from solution and frozen solution EPR spectra.
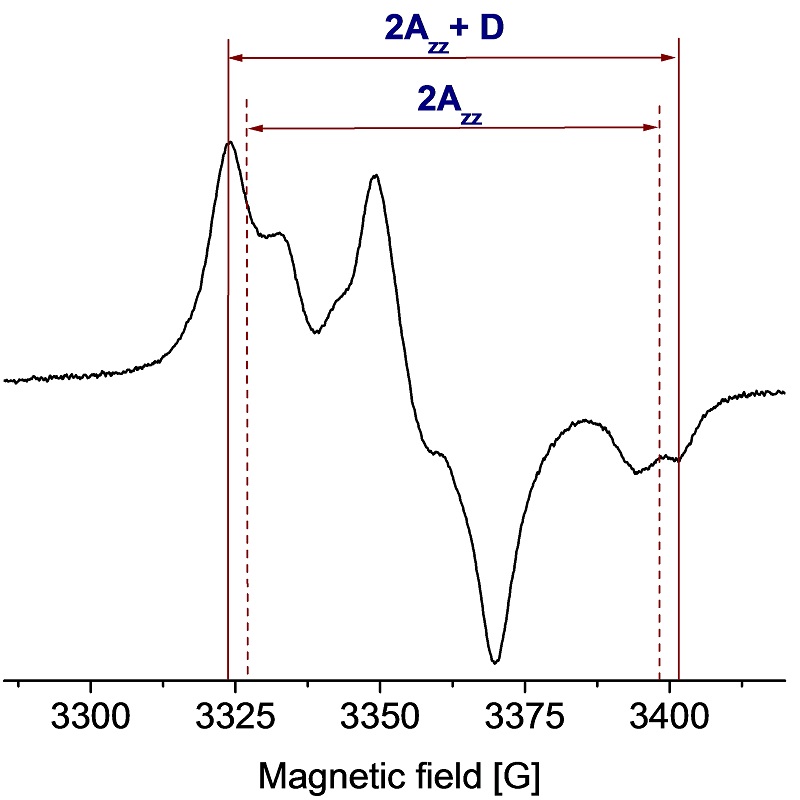
EPR spectrum of DNP agent (bis-TEMPO biradical)
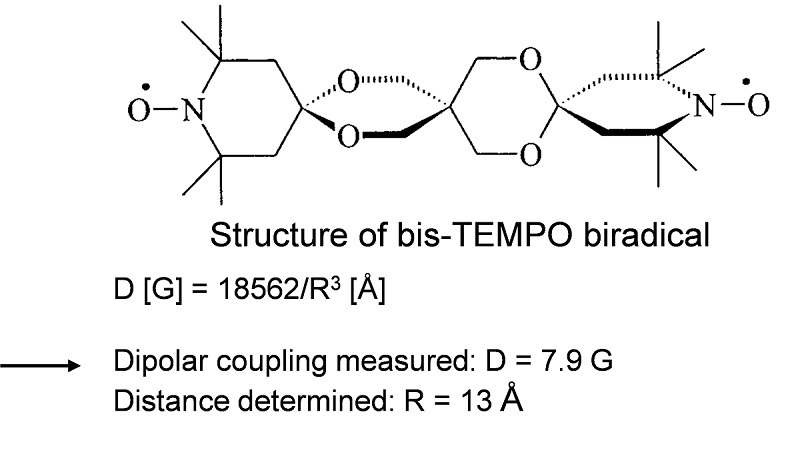
Data courtesy of Prof. Thomas Prisner, University of Frankfurt (Angew. Chem. Int. Ed., 2009, 48, 4996)
EPR in physic
Transition metals
Determination of Mg coordination in wurtzite thin films.
The transition-group, rare-earth and actinide ions are members of the 3d, 4d, 5d, 4f and 5f groups and are subject of a host of EPR investigations. One aspect that makes transition elements interesting subjects for study by EPR is their variable valence. For example, Zn1xMgxO complex is a versatile functional material for oxide semiconductors and the atomic arrangement in the bulk and at the interfaces determines important properties of the oxides. EPR is used to determine the Mg coordination in heteroepitaxial wurtzite Zn1xMgxO:Mn thin films.
Experimental and simulated EPR spectra at 297 K of Zn0.99Mg0.01O:Mn (pO2 = 0.016 mbar, cMn = 0.05%) thin film sample G5189 for B?c (top) and BIIc (bottom). Asterisks indicate signals of Fe3+ and Cr3+ impurities in the sapphire substrate.
The transition-group, rare-earth and actinide ions are members of the 3d, 4d, 5d, 4f and 5f groups and are subject of a host of EPR investigations. One aspect that makes transition elements interesting subjects for study by EPR is their variable valence. For example, Zn1xMgxO complex is a versatile functional material for oxide semiconductors and the atomic arrangement in the bulk and at the interfaces determines important properties of the oxides. EPR is used to determine the Mg coordination in heteroepitaxial wurtzite Zn1xMgxO:Mn thin films.
Experimental and simulated EPR spectra at 297 K of Zn0.99Mg0.01O:Mn (pO2 = 0.016 mbar, cMn = 0.05%) thin film sample G5189 for B?c (top) and BIIc (bottom). Asterisks indicate signals of Fe3+ and Cr3+ impurities in the sapphire substrate.
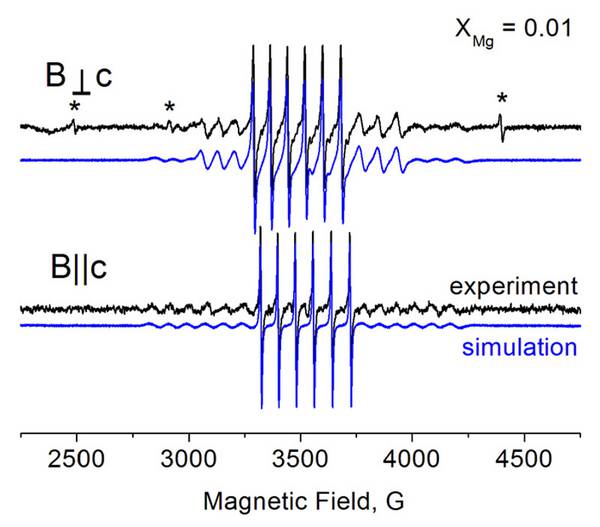
Data courtesy Dr. Andreas Pöppl, Universitӓt Leipzig (J. Mater. Chem. C, 2015, 3, 11918)
EPR in industry
Oxidative stability of foods and beverages
Oxidative stability of olive oil via quantitative EPR analysis.
The oxidative stability is a major problem in food related industries and is affected by a number of factors, such as oxygen, temperature, presence of metals and light. For example, extra virgin olive oil (EVOO) oxidation is of particular interest due to the complexity of its distribution channels around the world and the fact that it is an individually packaged product (its final quality reflects either positively or negatively on the producer). The resistance of EVOO to oxidation is related to the high levels of monounsaturated triacylglycerols and the presence of natural phenolic antioxidants. EPR is a useful tool to detect free radicals and to determine the level of free radical formation in olive oil during forced oxidation at different temperatures. Application of EPR to foods can reveal important information about radical reactions that may be responsible for food qualities and deterioration.
The oxidative stability is a major problem in food related industries and is affected by a number of factors, such as oxygen, temperature, presence of metals and light. For example, extra virgin olive oil (EVOO) oxidation is of particular interest due to the complexity of its distribution channels around the world and the fact that it is an individually packaged product (its final quality reflects either positively or negatively on the producer). The resistance of EVOO to oxidation is related to the high levels of monounsaturated triacylglycerols and the presence of natural phenolic antioxidants. EPR is a useful tool to detect free radicals and to determine the level of free radical formation in olive oil during forced oxidation at different temperatures. Application of EPR to foods can reveal important information about radical reactions that may be responsible for food qualities and deterioration.
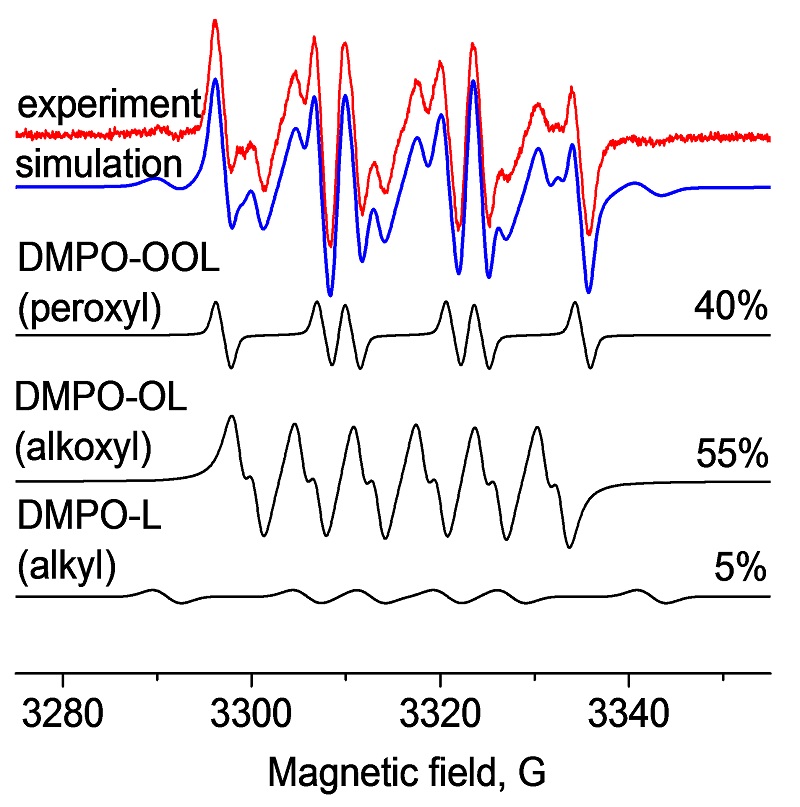
PR spectrum detected after addition of spin trap (DMPO) to an olive oil sample at 40 C.Experimental spectrum was simulated and each of the three radical components is presented as separate simulation
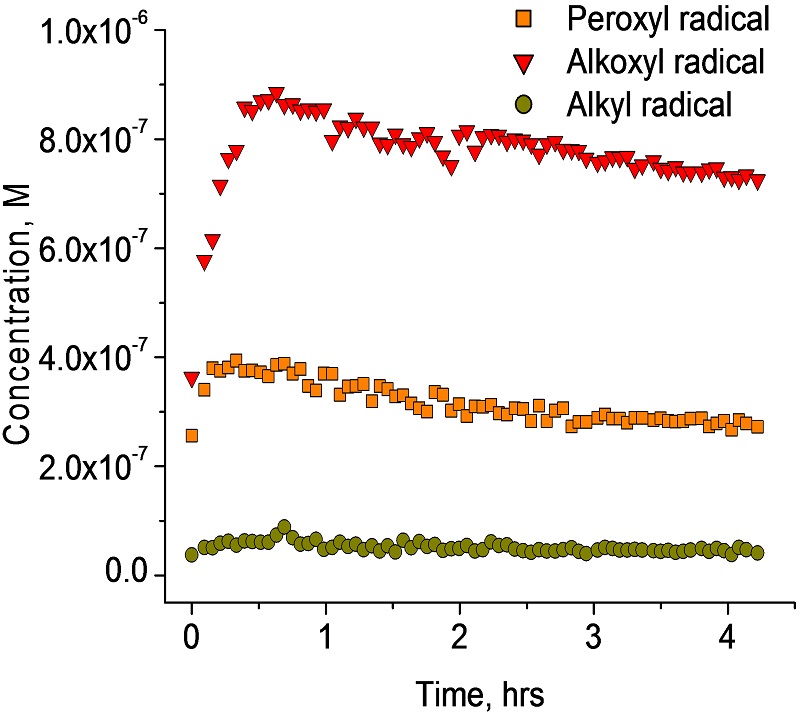
Concentrations of peroxyl, alkoxyl, alkyl DMPO-radical adducts,and the oxidized form of DMPO obtained in olive oil during oxidation at 40 C
Antioxidant capacity
DPPH scavenging assay to measure antioxidant activity in beverages.
Antioxidants play an important role as health protecting factor. Scientific evidence suggests that antioxidants reduce the risk for chronic diseases including cancer and heart disease. DPPH (2, 2-Diphenyl-1-picrylhydrazyl) is a free radical that is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors and to evaluate antioxidant activity. The DPPH assay method is based on the reduction of DPPH by antioxidants and is a rapid and simple method to measure antioxidant capacity of food and beverages.
Antioxidants play an important role as health protecting factor. Scientific evidence suggests that antioxidants reduce the risk for chronic diseases including cancer and heart disease. DPPH (2, 2-Diphenyl-1-picrylhydrazyl) is a free radical that is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors and to evaluate antioxidant activity. The DPPH assay method is based on the reduction of DPPH by antioxidants and is a rapid and simple method to measure antioxidant capacity of food and beverages.
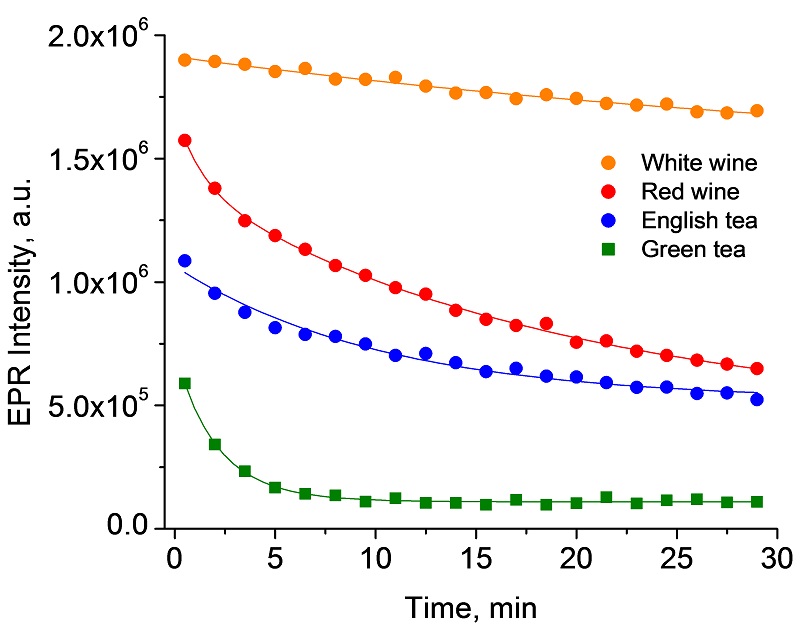
Antioxidant activity in wine and tea samples determined by the DPPH scavenging assay
Radiation dosimetry
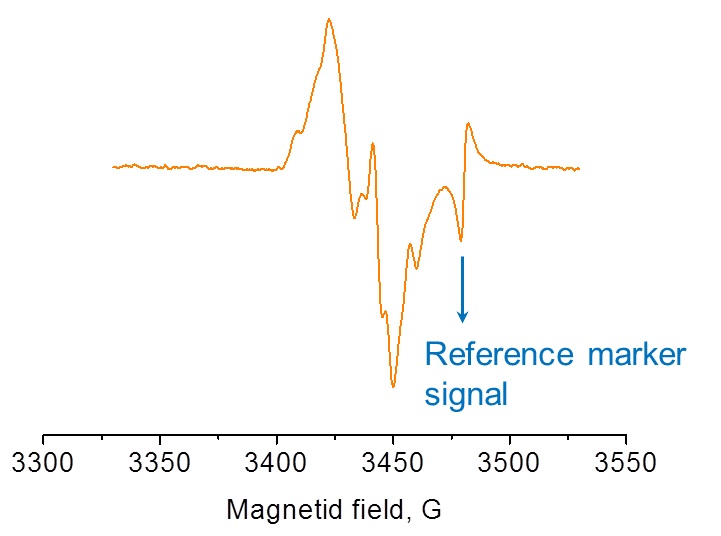
Alanine radical detected by EPR corresponds to the irradiation dose.
Alanine forms a very stable free radical when subjected to ionizing radiation. The alanine free radical yields an EPR signal that is dose dependent, yet is independent of the dose rate, energy type, and is relatively insensitive to temperature and humidity. Thus, alanine dosimetry is equally suited to gamma, e-beam, or x-ray irradiation facilities.
Alanine forms a very stable free radical when subjected to ionizing radiation. The alanine free radical yields an EPR signal that is dose dependent, yet is independent of the dose rate, energy type, and is relatively insensitive to temperature and humidity. Thus, alanine dosimetry is equally suited to gamma, e-beam, or x-ray irradiation facilities.
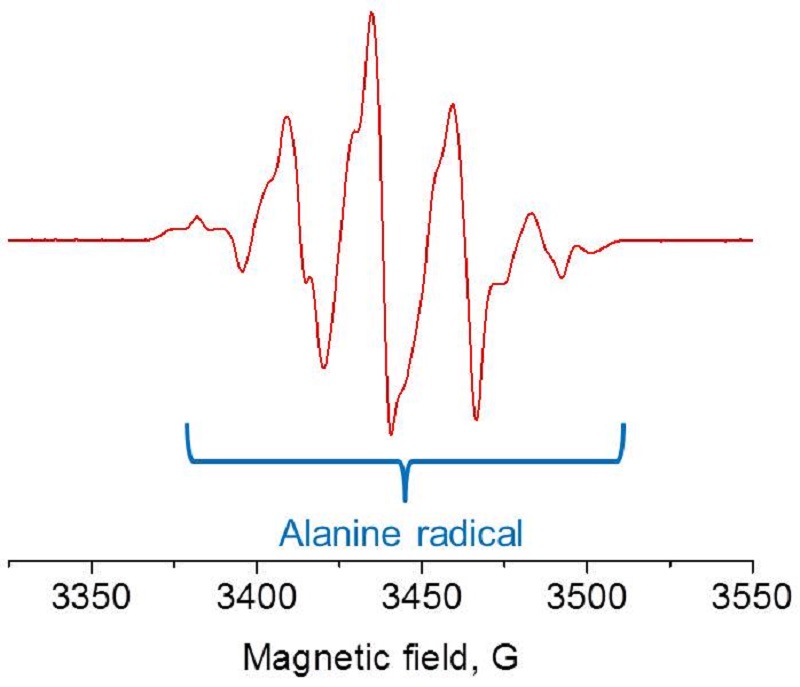
EPR signal of Alanine radical and the reference marker
Pharmaceutical analysis
Lactose radicals in the tablet filler causes enhanced degradation of the active pharmaceutical ingrediants (APIs)
EPR spectroscopy has a wide variety of applications within the analysis of pharmaceutical compounds. These include photodegradation and oxidation of APIs, the effects of sterilization techniques such as irradiation, interactions between APIs and excipients, etc. Excipients can initiate, propagate or participate in radical chemistry interactions which may compromise the effectiveness of a medication and studied by EPR. Lactose radicals originating from lactose monohydrate, used as a filler in the tablets, react with the API causing an enhanced degradation.
EPR spectroscopy has a wide variety of applications within the analysis of pharmaceutical compounds. These include photodegradation and oxidation of APIs, the effects of sterilization techniques such as irradiation, interactions between APIs and excipients, etc. Excipients can initiate, propagate or participate in radical chemistry interactions which may compromise the effectiveness of a medication and studied by EPR. Lactose radicals originating from lactose monohydrate, used as a filler in the tablets, react with the API causing an enhanced degradation.

Food science and beverages
Ionizing radiation of poultry and fruits creates very distinctive EPR spectra.
Food irradiation is used to reduce the health risk associated with food-borne pathogens and to prolong shelf life. In fact, ionizing radiation inhibits the division of microorganisms and creates radiolytic products as well as free radicals. In a dry environment these radicals are very stable. For example, irradiated poultry bones or fruits may contain a substantial amount of stable radicals which can be easily detected by EPR.
Food irradiation is used to reduce the health risk associated with food-borne pathogens and to prolong shelf life. In fact, ionizing radiation inhibits the division of microorganisms and creates radiolytic products as well as free radicals. In a dry environment these radicals are very stable. For example, irradiated poultry bones or fruits may contain a substantial amount of stable radicals which can be easily detected by EPR.
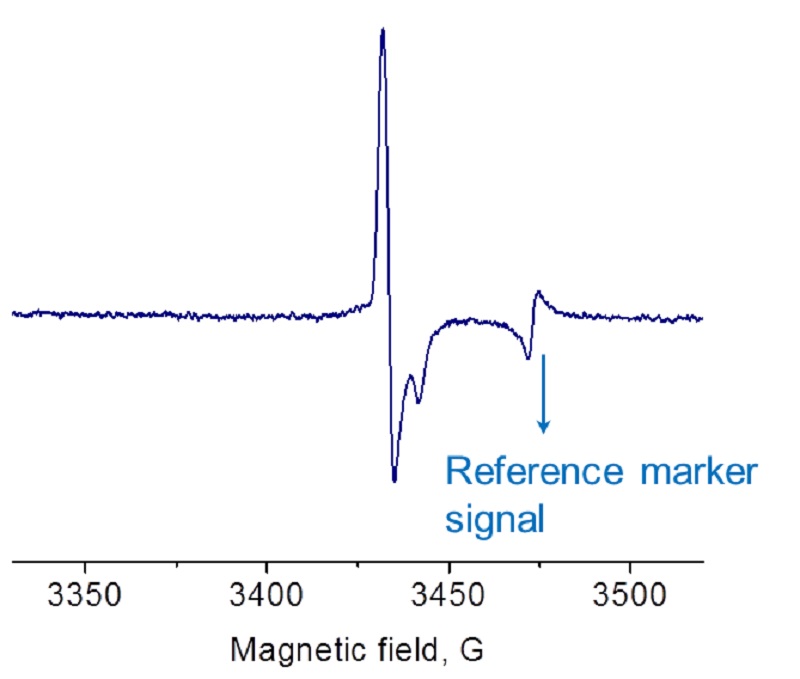
EPR spectrum of irradiated chicken bone
EPR spectrum of irradiated mango
Polymer research
Polyethylene radicals detected by EPR can predict premature failure of implants.
Ultra-high molecular weight polyethylene (UHMWPE) has been used as standard lining material in orthopedic implant industry. Oxidative degradation of the polymer caused by free radical formation can lead to premature aging and wear of the material and implant, causing a painful inflammation. EMXnano is capable of detecting and quantifying polyethylene radicals providing reliable and accurate measurements.
Ultra-high molecular weight polyethylene (UHMWPE) has been used as standard lining material in orthopedic implant industry. Oxidative degradation of the polymer caused by free radical formation can lead to premature aging and wear of the material and implant, causing a painful inflammation. EMXnano is capable of detecting and quantifying polyethylene radicals providing reliable and accurate measurements.
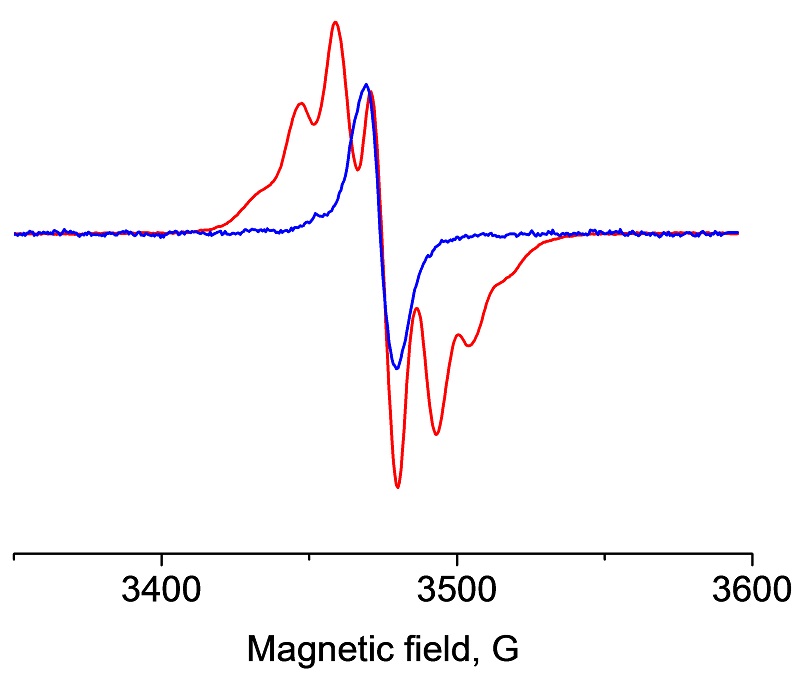
EPR spectra of two different polyethylene radicals
Data courtesy of Dr. Gavin Braithwaite, Cambridge Polymer Group
Diamond quality evaluation
EPR can detect the N3 and single substitution nitrogen centers in diamonds.
It is an unambiguous technique for quantifying nitrogen centers and hence provide a tool for color grading. It can also be used to distinguish between synthetic and natural diamonds.
It is an unambiguous technique for quantifying nitrogen centers and hence provide a tool for color grading. It can also be used to distinguish between synthetic and natural diamonds.
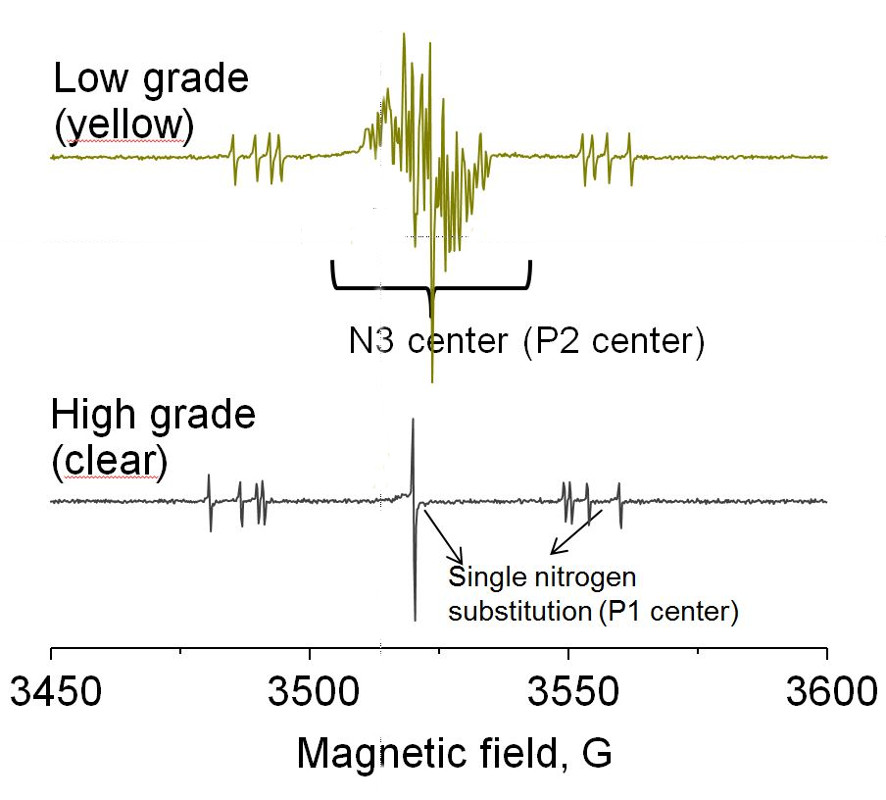
Room temperature EPR spectra of diamonds with different grades (colors)
EPR in education
As a company driven by innovation, Bruker recognizes the importance of a broad chemical education, and the importance of not only exposing students to a broad range of analytical techniques but also of teaching students the role each plays in chemical analysis. To introduce the upcoming generation of scientists to this powerful technique, Bruker has developed the ideal EPR teaching package that includes an easy-to-use instrument together with a suite of practical experiments, instructional guides and an introduction to the basic theory of EPR spectroscopy.
EMXnano teaching package includes:
As a company driven by innovation, Bruker recognizes the importance of a broad chemical education, and the importance of not only exposing students to a broad range of analytical techniques but also of teaching students the role each plays in chemical analysis. To introduce the upcoming generation of scientists to this powerful technique, Bruker has developed the ideal EPR teaching package that includes an easy-to-use instrument together with a suite of practical experiments, instructional guides and an introduction to the basic theory of EPR spectroscopy.
EMXnano teaching package includes:
- Easy-to-use X-band continuous wave EMXnano benchtop spectrometer fully optimized for a magnetic resonance teaching environment
- Introduction to the basic theory and practice of EPR spectroscopy
- Real life sample analysis in the classroom
- Quantitative EPR experiments
- Suite of principal experiments for teaching EPR data acquisition and processing skills (with full instructions)
- Collection of samples

microESR
microESR is a small, portable research grade instrument
The microESR is a small, portable research grade instrument. The spectrometer has a mass of only 10 kg and a 30.5 x 30.5 x 30.5 cm3 foot print. It can easily fit in a fume hood or glove box, or be transported to the field. It requires no special installation or regular maintenance.
The microESR is also an ideal teaching tool for undergraduate chemistry labs. This instrument enables classroom demonstrations of both simple topics such as free radicals in everyday life to far less intuitive subjects including electron density, spin-orbit coupling, spin-spin exchange, and forbidden transitions. The Education Package is a very good investment for Chemistry Departments as there are a wide range of labs and subjects that can be addressed with the microESR.
Education
Research Grade Teaching Tool
Chemistry
Reaction kinetics, free radical chemistry, catalysts, DNP
Biology/Biochemistry/Biomedical
Spin labeling, spin trapping, nitric oxides, ROS and RNS
Materials Science
Polymer degradation
Industry
Free radicals in polymers and polymerization, petrochemistry, thermoxidative breakdown of lubricants and fuel, real time analysis of additives, antioxidants in lubricants and fuels.
The microESR is a small, portable research grade instrument. The spectrometer has a mass of only 10 kg and a 30.5 x 30.5 x 30.5 cm3 foot print. It can easily fit in a fume hood or glove box, or be transported to the field. It requires no special installation or regular maintenance.
The microESR is also an ideal teaching tool for undergraduate chemistry labs. This instrument enables classroom demonstrations of both simple topics such as free radicals in everyday life to far less intuitive subjects including electron density, spin-orbit coupling, spin-spin exchange, and forbidden transitions. The Education Package is a very good investment for Chemistry Departments as there are a wide range of labs and subjects that can be addressed with the microESR.
- Operating Frequency: X-Band
- Continuous Wave
- Field Sweep Range: 500 G centered at g=2
- Spectrum simulation and fitting
- Easily run samples at liquid nitrogen temperature
Education
Research Grade Teaching Tool
Chemistry
Reaction kinetics, free radical chemistry, catalysts, DNP
Biology/Biochemistry/Biomedical
Spin labeling, spin trapping, nitric oxides, ROS and RNS
Materials Science
Polymer degradation
Industry
Free radicals in polymers and polymerization, petrochemistry, thermoxidative breakdown of lubricants and fuel, real time analysis of additives, antioxidants in lubricants and fuels.
Contact us
Syntech Innovation Co., Ltd.
388/5 Nuanchan Road, Nuanchan,
Buengkum, Bangkok 10230
388/5 Nuanchan Road, Nuanchan,
Buengkum, Bangkok 10230
0 2363 8585 (auto)
0 2363 8595
081 498 9939

3089611
Today
Yesterday
This Month
All days
569
1549
29319
3089611
Your IP: 216.73.216.80
2026-02-17 06:03






