Welcome to Syntech Innovation
Syntech Innovation Co., Ltd. is a company importing scientific instrument to a high standard and good quality products, which are recognized in the country and abroad. The company has team that is available in the installation maintenance and repair of scientific instrument. As well as consulting services in the creation and supply of laboratory science to suit the applications. Our products are divided into nine categories. The company is pleased to provide full service to our customers and sincerely hope that you will get the most for the success of our customers.

HIGHLIGHTS
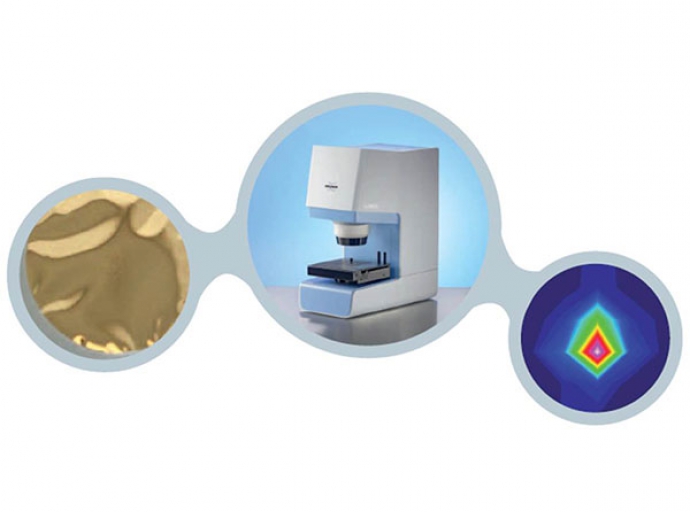
FT-IR microscopic analysis of micro-particles
FT-IR microscope the analysis of microparticles can be accomplished quickly and as a matter of routine.
...
Read More
Analysis of large samples with FT-IR
FT-IR microscope allows to examine large dimensioned samples without the need for a complex sample preparation.
...
Read More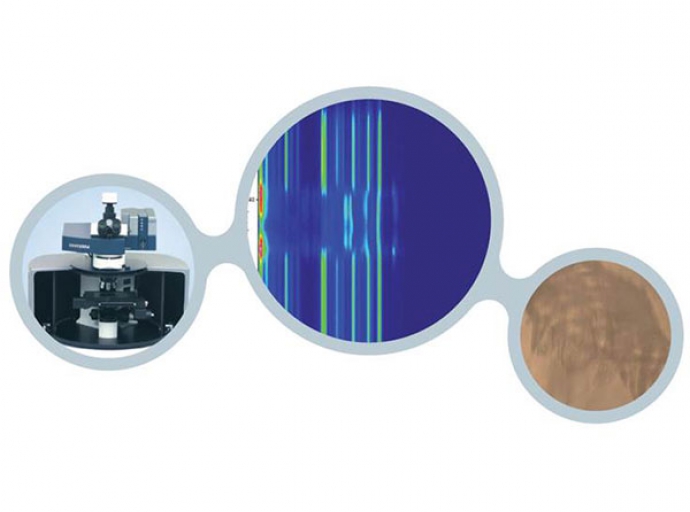
Depth profiling of complex samples
The Raman microscope is a powerful tool for the evaluation of complex samples and multilayer structures.
...
Read More